
HeartFlow FFRCT for estimating
fractional flow reserve from coronary CT
angiography
Medical technologies guidance
Published: 13 February 2017
© NICE 2017. All rights reserved.

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
Your responsibility
This guidance represents the view of NICE, arrived at after careful consideration of the evidence
available. When exercising their judgement, healthcare professionals are expected to take this
guidance fully into account. However, the guidance does not override the individual responsibility
of healthcare professionals to make decisions appropriate to the circumstances of the individual
patient, in consultation with the patient and/or guardian or carer.
Commissioners and/or providers have a responsibility to implement the guidance, in their local
context, in light of their duties to have due regard to the need to eliminate unlawful discrimination,
advance equality of opportunity, and foster good relations. Nothing in this guidance should be
interpreted in a way that would be inconsistent with compliance with those duties.
© NICE 2017. All rights reserved.
Page 2 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
Contents
4
5
5
7
8
8
12
19
19
21
21
26
27
27
27
© NICE 2017. All rights reserved.
Page 3 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
1
Recommendations
1.1
The case for adopting HeartFlow FFRCT for estimating fractional flow reserve
from coronary CT angiography (CCTA) is supported by the evidence. The
technology is non-invasive and safe, and has a high level of diagnostic accuracy.
1.2
HeartFlow FFRCT should be considered as an option for patients with stable,
recent onset chest pain who are offered CCTA as part of the NICE pathway on
angiography and revascularisation. For correct use, HeartFlow FFRCT requires
access to 64-slice (or above) CCTA facilities.
1.3
Based on the current evidence and assuming there is access to appropriate
CCTA facilities, using HeartFlow FFRCT may lead to cost savings of £214 per
patient. By adopting this technology, the NHS in England may save a minimum of
£9.1 million by 2022 through avoiding invasive investigation and treatment.
© NICE 2017. All rights reserved.
Page 4 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
2
The technology
Description of the technology
2.1
HeartFlow FFRCT (developed by HeartFlow) is coronary physiology simulation
software used for the qualitative and quantitative analysis of previously
acquired computerised tomography DICOM data. The software provides a non-
invasive method of estimating fractional flow reserve (FFR) using standard
coronary CT angiography (CCTA) image data. FFR is the ratio between the
maximum blood flow in a narrowed artery and the maximum blood flow in a
normal artery. FFR is currently measured invasively using a pressure wire
placed across a narrowed artery.
2.2
After a clinician decides to request a HeartFlow test, anonymised data from a
CCTA scan (of at least 64 slices) are sent from the local imaging system, by
secure data transfer to HeartFlow's central processing centre in the US. A case
analyst employed by the company then uses the image data to create 3D
computer models of the coronary arteries, incorporating coronary flow
characteristics. The results are presented in a report which is sent, by secure
data transfer, to the referring clinician within 48 hours. The report includes both
3D images of the coronary anatomy and calculated functional information,
including the estimated FFR values (known as FFRCT values). Clinicians can then
use the report to help guide the management of suspected coronary artery
disease.
2.3
HeartFlow FFRCT is intended for use in patients with stable, recent onset chest
pain and suspected angina. Because the safety and effectiveness of FFRCT
analysis has not been evaluated in other patient subgroups, HeartFlow FFRCT is
not recommended in patients who have an acute coronary syndrome or have
had a coronary stent, coronary bypass surgery or myocardial infarction in the
past month.
2.4
The company first received a CE mark in July 2011, covering all 1.X versions of
the technology, including the current version, 1.7.
2.5
HeartFlow FFRCT costs £700 per test. A higher price of £888 is used in the
company submission and assessment report. The cost was reduced in
May 2015.
© NICE 2017. All rights reserved.
Page 5 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
2.6
The claimed benefits of HeartFlow FFR
CT in the case for adoption presented by
the company were as follows:
Analysis is done using standard CCTA scans, without the need for additional imaging,
radiation or medication.
It provides the same accuracy in excluding coronary artery disease as CCTA, and
characterises the coronary arteries from both functional and anatomical perspectives,
differentiating between ischaemic and non-ischaemic vessels in a way that CCTA
cannot.
It allows physicians to evaluate anatomic coronary artery disease and accurately
determine which coronary lesions are responsible for myocardial ischaemia, avoiding
unnecessary invasive diagnostic or therapeutic procedures and related complications.
It reduces the need for revascularisation in patients after identifying anatomic stenosis
by invasive coronary angiography (ICA) alone, by more accurately identifying if those
stenoses are ischaemic.
It improves the diagnostic accuracy for coronary artery disease compared with CCTA
alone against the gold standard of invasive FFR, and provides both functional and
anatomic assessment of coronary arteries.
It has better diagnostic performance than CCTA alone, or other non-invasive or
invasive tests (such as nuclear myocardial perfusion, magnetic resonance perfusion,
stress echocardiography, exercise treadmill testing, invasive angiography or
intravascular ultrasound) for detecting and excluding coronary artery lesions that
cause ischaemia.
It reduces costs arising from inconclusive or inaccurate diagnostic tests.
It avoids staff and procedure costs for unnecessary ICAs.
It avoids staff and procedure costs for unnecessary interventions (such as angioplasty).
It provides a more effective use of high-cost invasive procedure suites, providing the
opportunity to reduce waiting times for these facilities and increase patient
turnaround.
© NICE 2017. All rights reserved.
Page 6 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
Current management
2.7
whom stable angina cannot be excluded by clinical assessment alone.
2.8
The guideline recommends offering 64-slice (or above) CCTA as the first-line
diagnosis test when clinical assessment indicates typical or atypical angina; or
non-anginal chest pain but 12-lead resting ECG has been done and indicates
ST-T changes or Q waves.
2.9
Subsequent diagnostic tests can be requested dependent on the CCTA results.
The guideline recommends offering non-invasive functional imaging for
myocardial ischaemia if 64-slice (or above) CCTA has shown coronary artery
disease of uncertain functional significance, or is non-diagnostic. Non-invasive
functional imaging includes:
myocardial perfusion scintigraphy with single-photon emission CT (MPS with SPECT)
stress echocardiography
first-pass contrast-enhanced MR perfusion
MR imaging for stress-induced wall motion abnormalities.
ICA should be offered as a second-line investigation when the results of non-invasive
functional imaging are inconclusive.
2.10
When ICA is used to determine the presence and severity of coronary stenosis,
it may be combined with the invasive measurement of FFR using a pressure
guidelines (such as those of the European Society of Cardiology and American
College of Cardiology) state that lesions with an FFR of 0.80 or less are
functionally significant and revascularisation may be considered.
© NICE 2017. All rights reserved.
Page 7 of 28
HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
3
Clinical evidence
3.1
The key clinical outcomes for HeartFlow FFRCT presented in the decision
problem were:
measures of diagnostic accuracy (sensitivity and specificity, positive and negative
likelihood ratios, area-under curve) using invasive fractional flow reserve (FFR) as the
reference standard
rates of diagnostic coronary angiography, percutaneous coronary intervention and
coronary artery bypass surgery
adverse events (test-related, major adverse cardiac events, radiation exposure and so
on)
quality of life
mortality.
Summary of diagnostic accuracy evidence
3.2
The company conducted a literature search on the diagnostic accuracy of FFRCT
and the existing tests in the current treatment pathway for patients with a 10%
to 90% pre-test likelihood of coronary artery disease, against a reference
standard of invasive FFR testing. This review identified 5 relevant meta-analysis
studies and 23 individual studies, 1 of which was unpublished. Based on the
22 published studies, and using FFR as the reference standard, the company
presented diagnostic accuracy per-patient results for HeartFlow FFRCT
compared with:
invasive coronary angiography (ICA)
single-photon emission CT (SPECT)
stress echocardiogram (ECHO)
magnetic resonance imaging (MRI)
coronary CT angiography (CCTA).
If there were multiple studies for a test, the company conducted a meta-analysis; for
© NICE 2017. All rights reserved.
Page 8 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
example, 3 studies were included in the meta-analysis for HeartFlow FFRCT (Koo et al.
2011, Min et al. 2012 and Nørgaard et al. 2014). The methodology and results of the
meta-analyses are reported as academic in confidence.
3.3
The external assessment centre (EAC) reviewed the company's selection of
studies and considered that although they addressed the scope in terms of the
comparators, reference test and outcomes, most included a mixture of patients
with both high (over 90%) and intermediate (10% to 90%) pre-test likelihoods of
disease. It also disagreed with the company's decision only to include studies
that provided FFR measurements in more than 75% of blood vessels. The EAC
considered this criterion not to be reflective of clinical practice, where visual
assessment is sometimes used before proceeding with FFR measurements. The
EAC also noted that this criterion did not reflect the company's proposed
changes to the clinical pathway, where CCTA would be used to decide if
HeartFlow FFRCT should be used.
3.4
To address these concerns, the EAC conducted a diagnostic literature search
using extra keywords related to comparators and outcomes. It included only
studies in which most patients had an intermediate pre-test likelihood of
disease. The EAC identified 7 diagnostic studies, including 3 presented by the
company (Bernhardt et al. 2012, Nørgaard et al. 2014 and Stuijfzand et al. 2014)
and 3 that the company had identified but excluded (Danad et al. 2013, Kajander
et al. 2010 and Ponte et al. 2014). Only 1 of these, Nørgaard et al. 2014,
involved HeartFlow FFRCT.
3.5
Nørgaard et al. (2014) reported on a multicentre study (the NXT trial) involving
2 UK centres, which compared HeartFlow FFRCT (v1.4) with CCTA for
diagnosing myocardial ischaemia in 254 patients with suspected stable
coronary artery disease scheduled to have ICA. Most patients in the study (87%)
were considered to have an intermediate likelihood of coronary artery disease.
Invasive FFR was measured in all vessels (n=484). The study reported the
diagnostic performance of HeartFlow FFRCT and CCTA for diagnosing ischaemia
compared with FFR measured during ICA as the reference standard. The
diagnostic accuracy of each test was presented on a per-patient and a per-vessel
basis compared with the reference standard, an FFR value of ≤0.80. Per-vessel
FFRCT was correlated to FFR (Pearson's correlation coefficient 0.82, p>0.001),
with a slight underestimation of FFRCT compared with FFR. The authors
concluded that HeartFlow FFRCT can identify functionally significant coronary
© NICE 2017. All rights reserved.
Page 9 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
artery disease with high sensitivity and specificity. Furthermore, adding FFRCT
measurements to CCTA led to a marked increase in specificity.
3.6
The EAC identified 6 studies which both used the comparator tests and included
patients with an intermediate likelihood of coronary artery disease. Bernhardt
et al. (2012) compared the diagnostic performance of 1.5 T and 3 T MRI
scanners using FFR as a reference standard in 34 patients with stable angina
and suspected or known coronary artery disease. The authors studied an
intermediate-risk population with a mean PROCAM score of 42.7 (a risk
assessment metric which estimates the 10-year risk of developing a coronary
event). Ponte et al. (2014) compared the diagnostic accuracy of CCTA and MRI
for detecting functionally significant coronary artery disease in patients
referred with suspected coronary artery disease, using ICA with FFR as the
reference standard. The study included 95 patients with a 15% to 85% pre-test
likelihood of coronary artery disease. Stuijfzand et al. (2014) evaluated CCTA
and transluminal attenuation gradient compared with CCTA alone for
diagnosing functionally significant lesions, using invasive FFR as the reference
standard. The study included 85 patients (253 vessels) with an intermediate
likelihood of coronary artery disease. Neglia et al. (2015) assessed the accuracy
of several imaging techniques - CCTA, SPECT and ECHO - in 475 patients with
an intermediate likelihood of coronary artery disease. Danad et al. (2013)
evaluated the diagnostic accuracy of CCTA in 120 patients with suspected
coronary artery disease who had cardiac positron emission topography (PET),
CCTA and ICA. CCTA was done using a hybrid PET/CT scanner. Kajander et al.
(2010) evaluated the diagnostic accuracy of PET and CCTA in 107 patients with
a history of stable chest pain and a 30% to 70% pre-test likelihood of coronary
artery disease. All patients had ICA independently of the non-invasive imaging
results, and treatment decisions were based on both ICA and FFR.
3.7
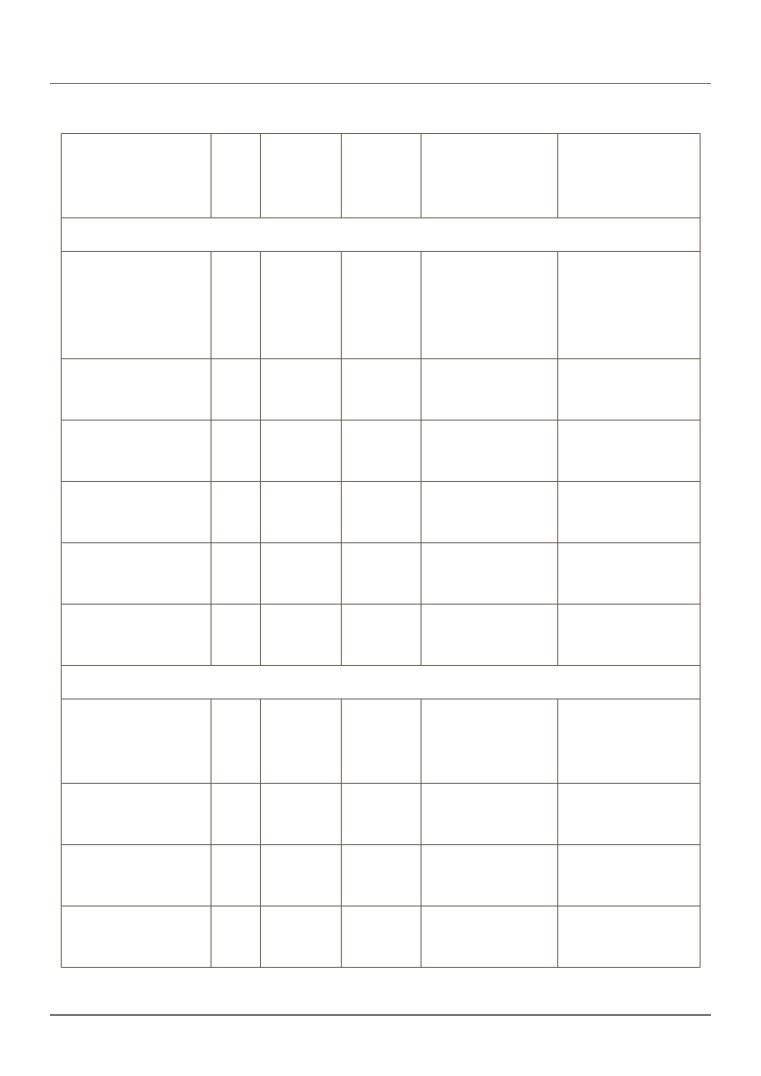
Table 1 summarises the EAC's analysis of diagnostic accuracy for
HeartFlow FFRCT and its comparators at both per-vessel and per-patient levels,
as shown in table 1. When there was more than 1 diagnostic accuracy study
available, the EAC conducted a meta-analysis.
© NICE 2017. All rights reserved.
Page 10 of 28
HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
Table 1 Diagnostic accuracy: HeartFlow FFRCT and comparator tests
Index test
N
Sensitivity
Specificity
Positive likelihood
Negative likelihood
ratio
ratio
(95% CI)
(95% CI)
(95% CI)
(95% CI)
Patient-based analysis
HeartFlow
254
0.86
0.79
4.07
0.18
FFRCT
0.77-0.93
0.72-0.85
3.02-5.49
0.10-0.31
(Nørgaard, 2014:
NXT trial)
CCTA
1,136
0.95
0.68
3.18
0.09
(6 studies)
0.92-0.97
0.65-0.71
1.56-6.47
0.05-0.16
ECHO
261
0.45
0.90
4.52
0.61
(Neglia, 2015)
0.33-0.57
0.85-0.94
2.74-7.45
0.49-0.76
ICA
254
0.64
0.83
3.70
0.44
(Nørgaard, 2014)
0.52-0.74
0.76-0.88
2.57-5.33
0.33-0.59
MRI
129
0.89
0.91
8.59
0.13
(2 studies)
0.78-0.95
0.82-0.97
4.12-17.9
0.07-0.26
SPECT
293
0.73
0.67
2.20
0.41
(Neglia, 2015)
0.63-0.81
0.60-0.74
1.74-2.79
0.29-0.57
Vessel-based analysis
HeartFlow
484
0.84
0.86
5.97
0.18
FFRCT
0.76-0.91
0.82-0.89
4.60-7.75
0.12-0.29
(Nørgaard, 2014)
CCTA
1,645
0.85
0.75
4.15
0.19
(4 studies)
0.81-0.89
0.73-0.77
2.38-7.23
0.12-0.32
ICA
484
0.55
0.90
5.56
0.50
(Nørgaard, 2014)
0.45-0.65
0.87-0.93
3.92-7.89
0.40-0.62
MRI
102
0.87
0.98
55.6
0.13
(Bernhardt, 2012)
0.72-0.96
0.92-1.00
7.92-390
0.06-0.30
© NICE 2017. All rights reserved.
Page 11 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
Abbreviations: CCTA, coronary CT angiography; CI, confidence interval; ECHO, stress
echocardiogram; FFRCT, fractional flow reserve CT; ICA, invasive coronary angiography; MRI,
magnetic resonance imaging; SPECT, single-photon emission CT.
3.8
The EAC considered that despite the limitations associated with patients having
a different reference test in some studies, all contributed to the decision
problem and provided data for synthesis. It judged that the Nørgaard (2014)
study had a low risk of bias for flow and timing, index and reference test. It
noted that there was a risk of patient selection bias because an inclusion
criterion was that patients had to have been referred for ICA, but it noted no
other risks of bias or applicability concerns. Although it acknowledged that
there were no studies directly comparing all the tests, it concluded that
HeartFlow FFRCT has:
similar sensitivity but higher specificity compared with CCTA
higher sensitivity but lower specificity compared with ECHO
similar sensitivity but lower specificity compared with MRI
higher sensitivity and specificity compared with SPECT.
Summary of clinical-effectiveness evidence
3.9
The company conducted a literature search for evidence on the clinical
outcomes specified in the decision problem for HeartFlow FFRCT, and the
existing treatments, against any comparator. It identified 16 studies of which
5 included HeartFlow FFRCT, 1 published (Guar et al. 2014) and 4 unpublished
(PLATFORM, Radiation FFRCT, Real World Usage FFRCT and FFRCT RIPCORD).
3.10
The EAC included extra intervention and comparator keywords and identified
11 studies, 4 of which had already been identified by the company: 2 published
studies (Hachamovitch et al. 2012 and Douglas et al. 2015) and 2 unpublished
studies. The EAC noted that only the 2 unpublished studies fully matched the
population, intervention, comparators and outcomes defined in the scope; the
other 9 included various comparators but not HeartFlow FFRCT. The
2 unpublished studies including HeartFlow FFRCT were PLATFORM (see
section 3.18) and Radiation FFRCT; the company provided both in the form of
interim results for the former and an abstract for the latter. Two studies (Real
© NICE 2017. All rights reserved.
Page 12 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
World Usage FFRCT and FFRCT RIPCORD) included HeartFlow FFRCT but were
excluded because they did not provide information on patients' pre-test
likelihood of coronary artery disease.
3.11
Radiation FFRCT is a single-centre modelling study, based in Canada,
investigating the potential effect of HeartFlow FFRCT on radiation dose
exposure and downstream clinical event rate. In the modelling, a clinical
pathway in which CCTA plus FFRCT was the initial diagnostic test was compared
with 3 clinical pathways instead utilising SPECT, ECHO or CCTA as initial
diagnostic tests. The model included 100 patients with suspected coronary
artery disease, 34% of whom had intermediate disease. Patients were stratified
into 3 categories of likelihood of disease: 50% low, 40% moderate and 10% high.
No clinical outcomes were measured in this modelled population. The primary
outcome was the estimated radiation dose and the secondary outcome was
death or myocardial infarction estimates at 1 year after the test. Of the
4 diagnostic pathways studied, ECHO had the lowest radiation dose (5.3 mSv)
but had a higher clinical event rate related to both false-positive and false-
negative findings. The FFRCT pathway had lower cumulative radiation exposure
(9.4 mSv) than SPECT (26.4 mSv) or CCTA (13.9 mSv) and also had the lowest
clinical adverse event rate for low and intermediate-risk patients. For high-risk
patients, the lowest clinical event rate was with ICA.
3.12
The PROMISE study (Douglas et al. 2015) is a US-based multicentre randomised
controlled trial involving over 10,000 patients, with a median follow-up of
25 months. Although the study did not include FFRCT, the EAC considered it
relevant to the decision problem because it provides further evidence on a
diagnostic pathway based on CCTA. Patients with a mean pre-test likelihood of
coronary artery disease of 53.3±21.4% were randomly assigned to either CCTA
or functional imaging as a first-line diagnostic test. The composite primary end
point was death, myocardial infarction, hospitalisation for unstable angina, or
major procedural complication. Secondary end points included invasive cardiac
catheterisation that did not show obstructive coronary artery disease and
radiation exposure. Results showed that 164 of 4,996 (3.3%) patients in the
CCTA group and in 151 of 5,007 (3.0%) in the functional testing group (adjusted
hazard ratio, 1.04; 95% confidence interval, 0.83 to 1.29; p=0.75) achieved the
primary end point. CCTA was associated with fewer catheterisations showing
no obstructive coronary artery disease than functional imaging (3.4% compared
with 4.3%, p=0.02), although more patients in the CCTA group had
© NICE 2017. All rights reserved.
Page 13 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
catheterisation within 90 days of randomisation (12.2% compared with 8.1%).
The median cumulative radiation exposure per patient was lower in the CCTA
group than in the functional testing group (10.0 mSv compared with 11.3 mSv),
but 32.6% of the patients in the functional testing group had no exposure. As
such, overall exposure was higher in the CCTA group (mean 12.0 mSv compared
with 10.1 mSv; p<0.001).
3.13
The EAC identified 9 published studies containing information on clinical
outcomes in comparator diagnostic technologies. Further information about
these studies can be found in the assessment report.
Chest pain guideline update: second literature search
3.14
During the assessment of HeartFlow FFRCT for this guidance, NICE updated its
investigating chest pain, it became necessary to update the evidence and cost
modelling for the HeartFlow FFRCT assessment. The EAC repeated the evidence
searches up to February 2016 and asked the company to identify any recent and
ongoing studies. In total, the EAC assessed 7 new studies, 6 of which included
HeartFlow FFRCT.
3.15
Tanaka et al. (2016) is a technical study on a subgroup of the NXT study
investigating the association between FFRCT and invasive FFR in coronary
arteries with serial lesions. The authors investigated patients (n=18 patients
and 18 vessels) with stable angina and clinically suspected coronary artery
disease. There was no clinical follow-up. The primary outcome was the per-
segment correlation between FFRCT and invasive FFR values, expressed as
translesional delta (the difference between the proximal and distal FFR
measurement of all sequential lesions). Values of translesional delta for FFR and
FFRCT were 0.10±0.09 and 0.09±0.10 in distal segments, and 0.17±0.10 and
0.22±0.13 in proximal segments respectively. The coefficient of correlation
between translesional delta FFR and FFRCT in each segment was 0.92 (p<0.001).
The authors concluded that translesional delta FFR is highly correlated with
FFRCT.
3.16
Thompson et al. (2015) investigated the diagnostic performance of FFRCT in
relation to patients' sex and age, using invasive FFR measurements as the
reference standard for a subgroup of the DeFACTO study. Previous evidence
© NICE 2017. All rights reserved.
Page 14 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
from DeFACTO was not considered eligible because it included patients with a
high pre-test likelihood of coronary artery disease (Min et al. 2012). Thompson
et al. (2015) was included because it reports results based on subgroup analyses
for age and sex. The baseline pre-test likelihood did not differ in statistical
significance within these subgroups, so it is not expected to bias the results. The
authors investigated 252 patients (407 vessels) with stable angina, clinically
suspected coronary artery disease and at least 1 coronary stenosis of 30% to
90%. For their analysis, the authors used a clinical rule that included all vessels
of diameter ≥2 mm and assigned an FFR value of 0.90 for vessels with stenoses
<30% and an FFR value of 0.50 for vessels with stenoses >90%. There was no
clinical follow-up. The primary outcome was per-patient and vessel diagnostic
performance of FFRCT. Using this clinical rule, diagnostic performance improved
in both sexes with no statistically significant differences between them. There
were no differences in the discrimination of FFRCT after application of the
clinical use rule when stratified by age ≥65 or <65 years. The authors concluded
that FFRCT had similar diagnostic accuracy and discriminatory power to FFR for
ischaemia detection in men and women irrespective of age using a cut-off point
of 65 years.
3.17
The other 4 studies on HeartFlow FFRCT looked at clinical outcomes. The
PLATFORM study (Douglas et al. 2015b and 2016) was presented to the
committee as academic in confidence in June 2015 (Douglas et al. 2015a). The
study included 584 patients recruited at 11 international centres. They were
prospectively assigned to have either functional imaging (n=287) or CCTA/
FFRCT (n=297). Each cohort was subdivided into 2 groups based on the
evaluation plan decided before enrolment in the study: non-invasive testing
(any form of stress testing or CCTA without FFRCT) or ICA (invasive testing).
3.18
Douglas et al. (2015b) report the study results at 3-month follow-up. The
primary end point was the percentage of patients with planned ICA in whom no
significant obstructive coronary artery disease (no stenosis ≥50% by core
laboratory quantitative analysis or invasive FFR <0.80) was found at ICA within
90 days. Secondary end points included a composite measure of major adverse
cardiac events consisting of death, myocardial infarction and unplanned
revascularisation, all of which were independently and blindly assessed. Among
patients with intended ICA (CCTA/FFRCT=193; functional imaging=187), no
obstructive coronary artery disease was found with ICA in 24 patients (12%) in
the CCTA/FFRCT arm and 137 patients (73%) in the functional imaging arm (risk
© NICE 2017. All rights reserved.
Page 15 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
difference 61%, 95% CI 53 to 69, p<0.0001). Among patients intended for non-
invasive testing, the rates of finding no obstructive coronary artery disease with
ICA were 13% in the CCTA/FFRCT arm and 6% in the functional imaging arm
(p=0.95). ICA was cancelled in 61% of patients after reviewing the CCTA/FFRCT
results. There were low numbers of MACE and vascular complications in all
groups.
3.19
Douglas et al. (2016) report outcomes from the same study at 1 year. The clinical
end points measured were MACE and MACE plus vascular events within
14 days of procedure. Quality of life and resource use outcomes were also
collected. There were 2 MACE events in each arm of the planned invasive group
(risk difference −0.03 [CI −8.6 to 8.5]) and 1 in the planned non-invasive group
(risk difference −1.00 [CI −12.7 to 10.7]). Cumulative 1-year radiation exposure
in patients in the intended invasive evaluation cohort was similar between the
usual care strategy (mean: 10.4±6.7 mSv) and CCTA/FFRCT-guided strategy
(mean: 10.7±9.6 mSv; p=0.21), whereas in the non-invasive testing cohort it was
higher in patients with a CCTA/FFRCT-guided strategy than usual care strategy
(mean: 9.6±10.6 mSv compared with 6.4±7.6 mSv, p<0.001). Functional status
and quality of life improved from baseline to 1-year follow-up in the planned
non-invasive group (p<0.001 for all measures), with greater improvements on
the EQ-5D in patients having CCTA/FFRCT compared with patients having
functional imaging (mean change: 0.12 for CCTA/FFRCT compared with 0.07 for
functional imaging, p=0.02).
3.20
Lu et al. (2015) used a subgroup analysis of the PROMISE trial (n=181) to
investigate the added value of FFRCT compared with CCTA in improving
efficiency of referral to ICA. End points for the subgroup analysis were rate of
revascularisation and ICA that did not show obstructive coronary artery disease
and MACE. Over a median follow-up of 25 months, the addition of FFRCT
increased the rate of ICA with revascularisation from 49% to 61%. The rate of
angiography without obstructive disease decreased from 27% to 11%. No
patient with FFRCT >0.80 had an adverse event which ICA would have
prevented.
3.21
Nørgaard (2016) reports on the real-world experience of using CCTA with
FFRCT as gatekeeper to ICA in patients with stable coronary artery disease and
intermediate-range coronary lesions (n=189). Patients were followed up for a
median of 12 months. The primary end point was the impact of FFRCT on further
© NICE 2017. All rights reserved.
Page 16 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
downstream diagnostic testing. Other end points were the agreement between
FFRCT and invasive FFR, and the short-term clinical outcome after FFRCT testing
defined as the occurrence of MACE (death and acute myocardial infarction) or
an angina episode leading to hospital admission or visit in the outpatient clinic.
The authors concluded that FFRCT testing is feasible in real-world scenarios
involving patients with intermediate-range coronary stenosis determined by
CCTA. They also concluded that implementing FFRCT for clinical decision-
making may influence the downstream diagnostic workflow, and patients with
an FFRCT >0.80 who are not referred for ICA have a favourable short-term
prognosis. The authors highlight that in patients with FFRCT ranging between
0.76 and 0.80, a non-negligible number of false-positive results may be
expected.
3.22
The EAC considered that the 1-year follow-up data from the PLATFORM study
supported the company's claims about resource use, rates of ICA and
percutaneous coronary intervention, and quality of life with HeartFlow FFRCT.
Additionally, the 1-year follow-up evidence from the PLATFORM supports the
company's claim that MACE outcomes are equivalent between the current
pathway and one that uses FFRCT, whereas the PROMISE study showed that
MACE outcomes at 1 year were equivalent between CCTA alone and functional
testing. The EAC also considered that the evidence from the PLATFORM study
showed higher 1-year radiation exposure in the HeartFlow FFRCT group in
patients intended for non-invasive evaluation. However, this is to be expected
because many patients in the non-invasive evaluation had a non-invasive test
which did not need the use of radiation. The EAC concluded that the submitted
evidence on clinical outcomes supports the value proposition of an FFRCT-
guided strategy compared with standard of care, mainly in patients with
planned invasive investigation, with equivalent results between FFRCT and
functional imaging in the non-invasive group.
Committee considerations
3.23
The committee considered that the evidence showed high diagnostic accuracy
and increased specificity with HeartFlow FFRCT compared with CCTA alone. It
also noted promising results from the PLATFORM study, in a population which
closely matches that in the scope. The evidence was sufficient to conclude that
HeartFlow FFRCT has a high diagnostic accuracy for coronary artery disease, and
© NICE 2017. All rights reserved.
Page 17 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
that its use has the potential to reduce the need for invasive coronary
investigations.
3.24
The committee considered the technology to be innovative and understood that
its adoption may serve to simplify a complex patient pathway. The committee
heard from clinical experts that they had confidence in the diagnostic accuracy
of HeartFlow FFRCT, and that it could provide an effective early rule-out test for
coronary artery disease. This would reduce the need for ICA and invasive FFR
measurement, and potentially reduce radiation exposure.
3.25
The committee understood that there are differences in the local
implementation of the patient pathway for diagnosing coronary artery disease.
It was advised by clinical experts that the choice of functional imaging test
depends on local access, available expertise and clinician preference. It heard
that although HeartFlow FFRCT has the potential to reduce the number of tests
that are done, the other non-invasive functional imaging tests that are part of
the current patient pathway offer different functionality and in some cases
provide additional information. Overall, the committee concluded that
HeartFlow FFRCT should be considered for use as a non-invasive investigation
for diagnosing angina in patients with stable, recent onset chest pain of
suspected cardiac origin, and that it provides the clinician with additional
functional information to determine which coronary lesions are responsible for
myocardial ischaemia. The committee considered that further clinical studies
would be helpful to clarify the wider applicability of HeartFlow FFRCT in routine
clinical practice.
3.26
The committee considered the evidence from the PLATFORM study to be most
relevant to the decision problem. It considered that the results demonstrate the
potential of FFRCT to avoid ICA and improve quality of life.
3.27
The committee discussed the relative importance of a per-patient or a per-
vessel diagnosis. It heard from experts that per-patient diagnostic accuracy was
more important for initial diagnosis, and that a per-vessel assessment provides
additional information to inform patient management. The committee
concluded that per-patient level figures were the most reliable and relevant to
the diagnosis of coronary artery disease.
© NICE 2017. All rights reserved.
Page 18 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
4
NHS considerations
System impact
4.1
The company's claimed system benefits included reduced costs from fewer
inconclusive or inaccurate diagnostic tests and avoidance of unnecessary staff
and procedure costs. It claimed that this would lead to more effective use of
invasive procedure suites.
4.2
The company confirmed that, with specific reference to the updated guideline
HeartFlow FFRCT (to inform management following a positive coronary CT
angiography [CCTA] result) was unchanged.
4.3
Conservative estimates by the NICE resource impact assessment team suggest
that by 2021/22, when fully implemented, HeartFlow FFRCT will potentially be
used in around 40,000 patients a year. This would equate to national savings of
at least £9.1 million a year.
4.4
During selection and routing, the committee asked for additional information on
compliance with data protection legislation, and the reproducibility of
HeartFlow FFRCT analysis, especially in the face of an increasing workload which
might be expected to arise from adoption of the technology in the NHS. The
external assessment centre (EAC) produced a technical report that concluded:
The company has a quality assurance process in place that fulfils data quality needs.
This includes checks by different team members, and the separation of tasks to ensure
that no single analyst is fully responsible for a diagnosis. After the procedure, a more
experienced analyst reviews the process, focusing mainly on areas of stenosis. Expert
clinician advice is also available should it be needed.
Although the analytical process is largely automated, any part of it can be manually
changed by the analyst. This may affect the fractional flow reserve CT (FFRCT)
estimate. Manual editing is part of the quality assurance process, negating the risk of
spurious results generated from the automated analysis. Gaur et al. (2014) suggest
that reproducibility is within acceptable 95% confidence interval limits of agreement.
FFRCT reproducibility was found to be equivalent to invasive FFR reproducibility.
© NICE 2017. All rights reserved.
Page 19 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
The reproducibility of outlining the coronary artery lumen, part of the FFRCT
computation analysis, decreases in the distal portion of the vessel (Gage repeatability
and reproducibility=29.4%). This could be a result of different factors including lower
CT quality, lower CT resolution, smaller vessel diameter at the distal end and higher
disease burden.
The company monitors FFRCT reproducibility by re-processing 5% of its case volume on
a weekly basis. The company has confirmed that this has shown a reproducibility rate
consistent with the literature (Gaur et al. 2014).
The company fulfils regulatory approval standards for data confidentiality and
integrity protection for remote processing. It offers NHS customers the option to
upload fully anonymised DICOM data to comply with UK data protection law.
Committee considerations
4.5
The committee was satisfied with the EAC's conclusions on reproducibility (see
section 4.4). It accepted that the company has protocols in place to manage an
increased demand for HeartFlow FFRCT.
4.6
The committee considered the protection and oversight of data transferred
during the administration of HeartFlow FFRCT to be an important factor in the
device's adoption. The committee was satisfied, on the basis of the information
available, that the company's data transfer protocols meet regulatory
requirements. The committee noted that patients should be informed when
sending personal data outside the European Economic Area with
HeartFlow FFRCT, and that it may be necessary to obtain written consent.
4.7
The committee considered the availability of CCTA facilities. It understood that
the cost model assumed access to CCTA facilities, but heard from experts that
access to CCTA varies across the NHS despite recommendations in NICE's
previous guideline on chest pain. Furthermore, because CT scanners are used
for many purposes, a constraint currently exists with regard to both the
availability of scanners and scanning time. The committee heard from experts
that a sizable investment would be needed for the wider implementation of
HeartFlow FFRCT, but acknowledged that this consideration was beyond the
scope of the current assessment. It understood that adopting 64-slice CCTA
was ongoing in the NHS, in line with the recommendations in the previous NICE
guideline on chest pain.
© NICE 2017. All rights reserved.
Page 20 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
5
Cost considerations
Cost evidence
5.1
The company conducted a search of the health economics literature on
HeartFlow FFRCT and the comparators specified in the decision problem. They
identified a total of 174 studies, 24 of which it considered relevant to the
decision problem.
5.2
The external assessment centre (EAC) reviewed this search, and considered that
most of the studies included neither an appropriate patient population nor a
treatment pathway. Only 1 published study, Rajani et al. (2015), was considered
by the EAC to be relevant to the decision problem. It conducted a further review
of the literature up to February 2016 and identified an additional relevant
published study, Hlatky et al. (2015).
5.3
Rajani et al. (2015) was a single-centre retrospective cost analysis of
410 patients referred to a rapid-access chest pain clinic in Guy's and St Thomas'
Hospital, London, from April 2012 to March 2013. Patients were grouped into
pre-test likelihood categories and diagnostic imaging was done based on
standardised protocols as recommended in the previous NICE guideline on
chest pain. A standardised unit cost for each test and procedure was taken from
the NHS National Tariff 2013/14. A decision-tree economic model was used to
evaluate the cost of 1,000 patients passing through the current treatment
pathway compared with the same 1,000 patients after incorporating
HeartFlow FFRCT. The authors found that introducing HeartFlow FFRCT to the
pathway resulted in cost savings of £200 per patient. The EAC noted that
although the derivation of costs in the study is explicit, details of the decision
model structure are unclear.
5.4
Hlatky et al. (2015) investigated the quality-of-life and economic outcomes of
fractional flow reserve CT (FFRCT) in the PLATFORM study (see section 3.17).
Cumulative medical costs were measured over 90 days for each patient by
multiplying a standardised cost weight for each medical resource by the number
of resources used by the patient. Medicare reimbursement rates (the national
average of technical and professional fees in the US) from 2015 were applied
because cost weights and online pharmacy costs were used for drugs. Patients
were prospectively assigned to either functional imaging (usual care, n=287) or
© NICE 2017. All rights reserved.
Page 21 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
coronary CT angiography (CCTA)/HeartFlow FFRCT (n=297). In the planed
invasive group, mean costs were $7,343 among the CCTA/FFRCT patients and
$10,734 among functional imaging patients (p<0.0001). In the planned non-
invasive group, mean costs were not significantly different (p=0.26) between
the CCTA/FFRCT patients ($2,679) and the functional imaging patients ($2,137).
Overall, each quality-of-life (EQ-5D) score improved at 90 days compared with
baseline in the study population (p<0.0001), and scores improved more in
CCTA/FFRCT patients than in functional imaging patients. In the invasive group,
quality-of-life improvements were similar in both arms.
5.5
Douglas et al. (2016) published data on the 1-year economic outcomes of FFRCT
in the PLATFORM study. Costs were calculated in the same manner as the
90-day results in Hlatky et al. (2015). In the planned invasive arm, the mean per-
patient cost was $8,127 in FFRCT patients and $12,145 for usual care patients
(p<0.0001). The cost savings at 1 year increased by 1.5% from the cost savings
at 90 days. In the non-invasive arm, mean costs were not significantly different
(p=0.82) between the FFRCT patients ($3,049) and the usual care patients
($2,579).
Economic model
5.6
The company presented a decision-tree model based on integrating
HeartFlow FFRCT into the existing diagnostic pathway at the time of its
submission. A theoretical population of 1,000 patients with suspected coronary
artery disease was allocated to either the current treatment pathway (based on
the previous NICE guideline on chest pain) or the company's revised pathway,
which included HeartFlow FFRCT. The cost consequences of the treatment
pathways were compared based on the mix of diagnostic technologies used in
each. The model had a 1-year time horizon, included the impact of different
testing strategies, and relevant clinical outcomes.
5.7
The proportion of patients eligible for CCTA as a first-line test and their
probability of having coronary artery disease were taken from Rajani et al.
(2015). In the model, 10% of patients were assumed to be ineligible for invasive
coronary angiography (ICA), have an inconclusive CCTA result and have an
uncertain single-photon emission CT (SPECT) result.
© NICE 2017. All rights reserved.
Page 22 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
5.8
The diagnostic accuracy of HeartFlow FFR
CT and its comparators in the
company's model were based on per-patient level results reported in selected
papers, as follows:
HeartFlow FFRCT: sensitivity 86%, specificity 79% (Nørgaard et al. 2014)
SPECT: sensitivity 76%, specificity 38% (Melikian et al. 2010)
CCTA: sensitivity 94%, specificity 48% (Meijboom et al. 2008)
ICA: sensitivity 69%, specificity 67% (Meijboom et al. 2008).
The cost of HeartFlow FFRCT (£888) was based on the company's original list price.
Costs for comparator tests were based on 2014/15 hospital resource group (HRG)
tariffs, as follows:
SPECT: £220 (HRG code RA37Z, nuclear medicine category 3)
CCTA: £136 (HRG code RA14Z, CT scan, more than 3 areas)
Calcium scoring: £77 (HRG code RA08Z, CT scan, 1 area, no contrast)
ICA: £1,241 (HRG code EA36A, catheter 19 years and over)
Percutaneous coronary intervention (PCI): £2,832 (weighted average of 2 tariffs,
assuming that 25% of patients needing PCI will need more than 2 stents. HRG codes
EA31Z [£2,704] and EA49Z [£3,216]).
5.9
The company's base-case results reported an average per-patient cost of
£2,239 using the current pathway and £2,080 using the adapted pathway with
HeartFlow FFRCT, representing an average saving of £159 per patient.
5.10
The company conducted 1-way sensitivity analyses on the sensitivity and
specificity of HeartFlow FFRCT and the comparator tests, as well as the costs of
HeartFlow FFRCT. The analyses showed that HeartFlow FFRCT continued to be
cost saving until its price reached £1,126. With regard to changes in the
sensitivity and specificity, HeartFlow FFRCT remained cost saving for nearly all
the values tested when considered in the context of the entire patient
population.
© NICE 2017. All rights reserved.
Page 23 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
Revisions by the external assessment centre
5.11
company economic model. In this context, the EAC assumed that
HeartFlow FFRCT would be used following an initial CCTA, and that non-invasive
functional imaging tests would subsequently be used only if the CCTA result
were uncertain or non-diagnostic. The EAC reviewed the parameters and costs
used in the company's model. It revised the company's sensitivity and specificity
parameters for the comparator diagnostic tests, based on its own analyses of
5.12
The EAC used the company's revised list price of £700 for HeartFlow FFRCT,
instead of £888 as used in the company's model.
5.13
of all comparator tests except MRI, to ensure that they were consistent with
2014/15 reference costs. The cost of MRI was taken from the Payment by
Results tariff, because the chest pain guideline committee determined this to be
more representative of the true cost. These costs were as follows:
SPECT: £367 (RN21Z, myocardial perfusion scan, stress only )
CCTA: £122 (RD28Z, complex CT scan)
ECHO: £271 (EY50Z, complex echocardiogram)
ICA: £1,685 (EY43A to EY43F, standard cardiac catheterisation)
MRI: £515 (RA67Z, cardiac MRI scan, pre and post contrast)
PCI: £2,865 (weighted average of 2 tariffs, assuming that 25% of patients needing PCI
will need more than 2 stents. HRG codes EA31Z [£2,704] and EA49Z [£3,216]).
Includes an estimated annual cost of £33 for medication following a PCI [aspirin and
clopidogrel, British national formulary (2015)].
5.14
The EAC noted that the company's model did not include costs of drug therapy
estimated an annual drug treatment cost for these patients of £33 based on
British national formulary (2015) prescription costs for aspirin and clopidogrel,
and used a cost of £2,865 (PCI tariff with drug costs) in its revised model.
© NICE 2017. All rights reserved.
Page 24 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
5.15
The EAC included a cost for optimal medical therapy. It obtained expert advice
that optimal medical therapy usually consists of aspirin, statins, nitrates and
beta blockers. Based on this information it estimated an annual cost of £84
(aspirin, simvastatin, glyceryl trinitrate and propranolol hydrochloride) from the
British national formulary (2015) and used it in the revised model.
5.16
Using these updated assumptions, the EAC found a base-case cost saving of
£214 per patient for HeartFlow FFRCT compared with the current treatment
pathway for all functional imaging tests (SPECT, MRI and ECHO).
5.17
The EAC ran a number of sensitivity analyses, varying: the price of
HeartFlow FFRCT; the diagnostic accuracy of the functional imaging tests,
HeartFlow FFRCT, ICA and CCTA; and the proportion of uncertain CCTA and
functional imaging tests. It also used estimates of diagnostic accuracy for CCTA
HeartFlow FFRCT remained cost saving.
Committee considerations
5.18
The committee considered the cost modelling done by the EAC to be both
appropriate and plausible. The committee heard from experts that
percutaneous or surgical revascularisation is only offered to patients following
ICA, and sometimes a confirmatory invasive FFR. The availability of data from
HeartFlow FFRCT may help to plan treatment in individual vessels and patients.
© NICE 2017. All rights reserved.
Page 25 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
6
Conclusions
6.1
The committee concluded that the evidence suggests that HeartFlow FFRCT is
safe, has high diagnostic accuracy, and that its use may avoid the need for
invasive investigations.
6.2
The committee concluded that cost savings of £214 per patient are plausible
and likely to be realised in practice, providing that sites adopting
HeartFlow FFRCT have access to 64-slice (or above) coronary CT angiography.
© NICE 2017. All rights reserved.
Page 26 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
7
Committee members and NICE project team
Committee members
advisory committee of NICE.
Committee members are asked to declare any interests in the technology to be appraised. If it is
considered there is a conflict of interest, the member is excluded from participating further in that
evaluation.
and their declarations of interests, are posted on the NICE website.
NICE project team
Each medical technologies guidance topic is assigned to a team consisting of 1 or more health
technology analysts (who act as technical leads for the topic) and a technical adviser or senior
technical analyst.
Neil Hewitt
Technical analyst
Paul Dimmock
Technical analyst (evaluations)
Jae Long
Project manager
ISBN: 978-1-4731-2333-5
© NICE 2017. All rights reserved.
Page 27 of 28

HeartFlow FFRCT for estimating fractional flow reserve from coronary CT angiography (MTG32)
Accreditation
© NICE 2017. All rights reserved.
Page 28 of 28