Coronary Computed Tomography Angiography with
Page 1 of 23
Selective Noninvasive Fractional Flow Reserve
Medical Policy
An independent licensee of the
Blue Cross Blue Shield Association
Title:
Coronary Computed Tomography Angiography with
Selective Noninvasive Fractional Flow Reserve
Professional
Institutional
Original Effective Date: October 15, 2017
Original Effective Date: October 15, 2017
Revision Date(s): October 15, 2017
Revision Date(s): October 15, 2017
Current Effective Date: October 15, 2017
Current Effective Date: October 15, 2017
State and Federal mandates and health plan member contract language, including specific
provisions/exclusions, take precedence over Medical Policy and must be considered first in
determining eligibility for coverage. To verify a member's benefits, contact Blue Cross and
Blue Shield of Kansas Customer Service.
The BCBSKS Medical Policies contained herein are for informational purposes and apply only
to members who have health insurance through BCBSKS or who are covered by a self-insured
group plan administered by BCBSKS. Medical Policy for FEP members is subject to FEP medical
policy which may differ from BCBSKS Medical Policy.
The medical policies do not constitute medical advice or medical care. Treating health care
providers are independent contractors and are neither employees nor agents of Blue Cross
and Blue Shield of Kansas and are solely responsible for diagnosis, treatment and medical
advice.
If your patient is covered under a different Blue Cross and Blue Shield plan, please refer to the
Medical Policies of that plan.
Populations
Interventions
Comparators
Outcomes
Individuals:
Intervention of interest
Comparator of interest are:
Relevant outcomes include:
• With stable chest pain
are:
• Coronary computed
• Test accuracy
at intermediate risk of
• Noninvasive fractional
tomography angiography
• Test validity
coronary artery disease
flow reserve
without noninvasive
• Morbid events
being considered for
measurement following
fractional flow reserve
• Quality of life
invasive coronary
positive coronary
• Invasive coronary
• Resource utilization
angiography
computed tomography
angiography
• Treatment-related morbidity
angiography
• Other noninvasive
functional tests
DESCRIPTION
Invasive coronary angiography (ICA) is clinically useful in stable ischemic heart disease
(SIHD) when there is coronary artery obstruction that may benefit from revascularization.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 2 of 23
Selective Noninvasive Fractional Flow Reserve
However, many individuals currently undergoing ICA will not benefit from
revascularization. Therefore, if there are noninvasive alternatives to guide decisions
about the use of ICA to spare individuals from undergoing unnecessary ICA, there is
potential to improve health outcomes. Using noninvasive measurement of fractional flow
reserve as part of a noninvasive imaging strategy prior to ICA may be beneficial to avoid
the need for ICA.
OBJECTIVE
The objective of this policy is to evaluate the net health outcome when a noninvasive
imaging strategy using coronary computed tomography angiography with a noninvasive
assessment of fractional flow reserve is used to guide decisions about the use of invasive
coronary angiography in patients with stable chest pain and suspected stable ischemic
heart disease.
BACKGROUND
Stable Ischemic Heart Disease
Coronary artery disease (CAD) is a significant cause of morbidity and mortality and
various epidemiologic risk factors have been well studied. Evaluation of obstructive CAD
involves quantifying arterial stenoses to determine whether significant narrowing is
present. Lesions with stenosis more than 50% to 70% in diameter accompanied by
symptoms are generally considered significant. It has been suggested that coronary
computed tomography angiography (CCTA) or other noninvasive functional cardiac
testing may help rule out CAD and avoid invasive coronary angiography in patients with a
low clinical likelihood of significant CAD. However, invasive coronary angiographies
(ICAs) are frequently unnecessary in patients with suspected stable ischemic heart
disease (SIHD), as evidenced by low diagnostic yields for significant obstructive CAD. For
example, from a sample of over 132,000 ICAs, Patel et al (2010) found 48.8% of elective
ICAs performed in patients with stable angina did not detect obstructive CAD (left main
stenosis ≥50% or ≥70% in a major epicardial or branch >2.0 mm in diameter).1 ICA is
clinically useful when patients with stable angina have failed optimal medical therapy and
may benefit from revascularization. A noninvasive imaging test, performed prior to ICA
as a gatekeeper, that can distinguish candidates who may benefit from early
revascularization (eg, patients with unprotected left main stenosis ≥50% or
hemodynamically significant disease) from those unlikely to benefit could avoid
unnecessary invasive procedures and their potential adverse consequences. Moreover,
for the large majority of patients with SIHD, revascularization offers no survival
advantage over medical therapy; there are few who might benefit from ICA if they have
not first failed optimal medical therapy.2
Clinical Risk Prediction
The 2012 collaborative medical association guidelines for the diagnosis and management
of patients with stable heart disease list several class I recommendations on use of
noninvasive testing in patients with suspected SIHD.3 A class I recommendation indicates
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 3 of 23
Selective Noninvasive Fractional Flow Reserve
that a test should be performed. In general, patients with at least intermediate risk
(10%-90% risk by standard risk prediction instruments) are recommended to have some
type of test, the choice depending on interpretability of the electrocardiogram, capacity
to exercise, and presence of comorbidity.
Clinical prediction scores or models have been developed to help estimate the pretest
probability of CAD in individuals with stable chest pain. A commonly cited clinical
prediction model based on age, sex, and type of pain symptoms, originally developed by
Diamond and Forrester (1979),4 has been further studied and extended in a report by
Genders et al (2011)5 and compared to the Duke Clinical Score by Wasfy et al (2012).6
Versteylen et al (2011) published a comparison of clinical prediction results for the
Diamond and Forrester model, the Framingham risk score, the PROCAM risk score, and
the SCORE risk estimation model.7 Another model has been published by Min et al
(2015)8 and an online calculator developed by a CAD consortium.9,10
Gatekeepers to ICA
Imposing an effective noninvasive gatekeeper strategy with one or more tests before
planned ICA to avoid unnecessary procedures is compelling. The most important
characteristic of a gatekeeper test is its ability to accurately identify and exclude clinically
insignificant disease where revascularization would offer no potential benefit. From a
diagnostic perspective, an optimal strategy would result in few false-negative tests while
avoiding an excessive false-positive rate—it would provide a low posttest probability of
significant disease. Such a test would then have a small and precise negative likelihood
ratio and high negative predictive value. An effective gatekeeper would decrease the rate
of ICA while increasing the diagnostic yield (defined by the presence of obstructive CAD
on ICA). At the same time, there should be no increase in major adverse cardiac events.
A clinically useful strategy would satisfy these diagnostic performance characteristics and
impact the outcomes of interest. Various tests have been proposed as potentially
appropriate for a gatekeeper function prior to planned ICA, including CCTA, magnetic
resonance imaging, single-photon emission computed tomography, positron emission
tomography, and stress echocardiography. More recently, adding noninvasive
measurement of fractional flow reserve (FFR) using CCTA has been suggested,
combining functional and anatomic information.
Fractional Flow Reserve
Invasively measured FFR evaluates the severity of ischemia caused by coronary artery
obstructions and can predict when revascularization may be beneficial.11-13 FFR has not
been used as a diagnostic test for ischemic heart disease, but as a test to evaluate the
degree of ischemia caused by a stenosis.
Invasive FFR is rarely used in the United States to guide percutaneous coronary
intervention (PCI). For example, using the National Inpatient Sample, Pothineni et al
(2016) reported that 201,705 PCIs were performed in 2012, but just 21,365 FFR
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 4 of 23
Selective Noninvasive Fractional Flow Reserve
procedures.14 Assuming the intention of FFR is to guide PCI, it would represent just 4.3%
of PCI procedures. Whether noninvasively obtained FFR will influence decisions concerning
ICA, over and above anatomic considerations, is therefore important to empirically
establish.
Randomized controlled trials and observational studies have demonstrated that FFR-
guided revascularization can improve cardiovascular outcomes, reduce revascularizations,
and decrease costs.15 For example, the Fractional Flow Reserve versus Angiography for
Multivessel Evaluation (FAME) trial randomized 1005 patients with multivessel disease
and planned PCI.13,16 At 1 year, compared with PCI guided by angiography alone, FFR-
guided PCI reduced the number of stents placed by approximately 30%—followed by
lower rates (13.2% vs 18.3%) of major cardiovascular adverse events (myocardial
infarction, death, repeat revascularization) and at a lower cost. The clinical benefit
persisted through 2 years, although by 5 years events rates were similar between
groups.17
European guidelines (2013) for stable CAD have recommended that FFR be used “to
identify hemodynamically relevant coronary lesion(s) when evidence of ischaemia is not
available” (class Ia), and “[r]evascularization of stenoses with FFR <0.80 is
recommended in patients with angina symptoms or a positive stress test.”18 Guidelines
(2014) have also recommended using “FFR to identify haemodynamically relevant
coronary lesion(s) in stable patients when evidence of ischaemia is not available” (class
Ia recommendation).19 U.S. guidelines (2012) have stated that an FFR of 0.80 or less
provides level Ia evidence for revascularization for “significant stenoses amenable to
revascularization and unacceptable angina despite guideline directed medical therapy.”3
In addition, the importance of FFR in decision making appears prominently in the 2017
appropriate use criteria for coronary revascularization in patients with SIHD.20
Measuring FFR during ICA can be accomplished by passing a pressure-sensing guidewire
across a stenosis. Coronary hyperemia (increased blood flow) is then induced and
pressure distal and proximal to the stenosis is used to calculate flow across it. FFR is the
ratio of flow in the presence of a stenosis to flow in its absence. FFR levels less than 0.75
to 0.80 are considered to represent significant ischemia while those 0.94 to 1.0 normal.
Measurement is valid in the presence of serial stenoses, is unaffected by collateral blood
flow,21 and reproducibility is high.22 Potential complications include adverse events
related to catheter use such as vessel wall damage (dissection); the time required to
obtain FFR during a typical ICA is less than 10 minutes.
FFR using CCTA requires at least 64-slice CCTA and cannot be calculated when images
lack sufficient quality23 (11% to 13% in recent studies24-27), eg, in obese individuals (eg,
body mass index, >35 kg/m2). The presence of dense arterial calcification or an
intracoronary stent can produce significant beam-hardening artifacts and may preclude
satisfactory imaging. The presence of an uncontrolled rapid heart rate or arrhythmia
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 5 of 23
Selective Noninvasive Fractional Flow Reserve
hinders the ability to obtain diagnostically satisfactory images. Evaluation of the distal
coronary arteries is generally more difficult than visualization of the proximal and mid-
segment coronary arteries due to greater cardiac motion and the smaller caliber of
coronary vessels in distal locations.
Noninvasive FFR Measurement
FFR can be modeled noninvasively using images obtained during CCTA28—so-called
fractional flow reserve using coronary computed tomography angiography (FFR-CT;
HeartFlow software termed FFRCT; Siemens cFFR) using routinely collected CCTA imaging
data. The process involves constructing a digital model of coronary anatomy and
calculating FFR across the entire vascular tree using computational fluid dynamics. FFR-
CT can also be used for “virtual stenting” to simulate how stent placement would be
predicted to improve vessel flow.29
Only the HeartFlow FFRCT software has been cleared by the U.S. Food and Drug
Administration. Imaging analyses require transmitting data to a central location for
analysis, taking 1 to 3 days to complete. Other prototype software is workstation-based
with onsite analyses. FFR-CT requires at least 64-slice CCTA and cannot be calculated
when images lack sufficient quality23 (11% to 13% in recent studies24-27), eg, in obese
individuals (eg, body mass index, >35 kg/m2).
REGULATORY STATUS
In November 2014, FFRCT simulation software (HeartFlow) was cleared for marketing by
the U.S. Food and Drug Administration (FDA) through the de novo 510(k) process (class
II, special controls; FDA product code: PJA). In January 2016, the FFRCT v2.0 device was
cleared through a subsequent 510(k) process.
HeartFlow FFRCT postprocessing software is cleared “for the clinical quantitative and
qualitative analysis of previously acquired Computed Tomography [CT] DICOM [Digital
Imaging and Communications in Medicine] data for clinically stable symptomatic patients
with coronary artery disease. It provides FFRCT [fractional flow reserve using coronary
computed tomography angiography], a mathematically derived quantity, computed from
simulated pressure, velocity and blood flow information obtained from a 3D computer
model generated from static coronary CT images. FFRCT analysis is intended to support
the functional evaluation of coronary artery disease. The results of this analysis [FFRCT]
are provided to support qualified clinicians to aid in the evaluation and assessment of
coronary arteries. The results of HeartFlow FFRCT are intended to be used by qualified
clinicians in conjunction with the patient’s clinical history, symptoms, and other
diagnostic tests, as well as the clinician’s professional judgment.”
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 6 of 23
Selective Noninvasive Fractional Flow Reserve
POLICY
A. The use of noninvasive fractional flow reserve following a positive coronary
computed tomography angiography may be considered medically necessary to
guide decisions about the use of invasive coronary angiography in patients with
stable chest pain at intermediate risk of coronary artery disease (ie, suspected or
presumed stable ischemic heart disease).
B. The use of noninvasive fractional flow reserve not meeting the criteria outlined
above is considered experimental / investigational.
Policy Guidelines
Fractional flow reserve using coronary computed tomography angiography requires at
least 64-slice coronary computed tomography angiography and cannot be calculated
when images lack sufficient quality (HeartFlow, 2013) (11% to 13% in recent studies;
Koo et al, 2011; Min et al, 2012; Nakazato et al, 2013; Nørgaard et al, 2014), eg, in
obese individuals (eg, body mass index, >35 kg/m2). The presence of dense arterial
calcification or an intracoronary stent can produce significant beam-hardening artifacts
and may preclude satisfactory imaging. The presence of an uncontrolled rapid heart rate
or arrhythmia hinders the ability to obtain diagnostically satisfactory images. Evaluation
of the distal coronary arteries is generally more difficult than visualization of the proximal
and mid-segment coronary arteries due to greater cardiac motion and the smaller caliber
of coronary vessels in distal locations.
RATIONALE
The most recent literature review was performed through April 11, 2017, to identify literature
assessing the potential impact of noninvasive imaging, particularly focusing on use of coronary
computed tomography angiography (CCTA) and noninvasive fractional flow reserve (FFR), to
guide use of invasive coronary angiography (ICA) in patients with stable chest pain at
intermediate risk of coronary artery disease (CAD; ie, suspected or presumed stable ischemic
heart disease [SIHD]) being considered for ICA. HeartFlow also submitted a list of publications
and materials for review.
Assessment of a diagnostic technology typically focuses on 3 categories of evidence: (1) its
technical performance (test-retest reliability or interrater reliability); (2) diagnostic accuracy
(sensitivity, specificity, and positive and negative predictive value) in relevant populations of
patients; and (3) clinical utility demonstrating that the diagnostic information can be used to
improve patient outcomes.
CCTA with Selective Noninvasive FFR
Clinical Context and Test Purpose
The purpose of selective noninvasive fractional flow reserve using coronary computed
tomography angiography (FFR-CT) in patients with stable chest pain who have suspected SIHD
and who are being considered for ICA is to select patients who may be managed safely with
observation only, instead of undergoing ICA in the short term.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 7 of 23
Selective Noninvasive Fractional Flow Reserve
The following PICOTS were used to select literature to inform this review.
Patients
The population of interest includes patients with stable chest pain at intermediate risk of CAD (ie,
with suspected or presumed SIHD) who are being considered for ICA. Patients may have
undergone prior noninvasive testing and been treated for presumed stable angina.
Interventions
The intervention of interest is CCTA with selective FFR-CT when CCTA shows evidence of
coronary artery stenosis.
Comparators
The comparator of interest is CCTA may be performed alone without FFR-CT. Individuals may
proceed directly to ICA. Conventional noninvasive imaging tests providing functional information,
including myocardial perfusion imaging (MPI) using single-photon emission computed
tomography (SPECT), stress echocardiography (SECHO), and cardiac positron emission
tomography (PET), may be used prior to ICA. Cardiovascular magnetic resonance imaging (MRI)
is also an option.
Outcomes
The final outcomes of interest include ICA rates, ICA without obstructive CAD, major adverse
cardiovascular events (MACE), and adverse events attributed to testing and treatment.
The intermediate outcome of interest is the ability of the test to distinguish clinically significant
CAD for which revascularization may provide benefit.
Timing
Rates of ICA and treatment-related morbidity are typically short-term (eg, ≤3 months). In
addition, rates of subsequent ICA, treatment-related morbidity, MACE, quality of life, and
resource utilization ascertained over a period of 1 to 3 years are also of interest.
Setting
The setting is a general cardiology practice for patients undergoing nonemergent chest pain
evaluation.
Technical Performance
Data supporting technical performance derive from the test-retest reliability of FFR-CT and
invasively measured FFR (reference standard). Other technical performance considerations were
summarized in the Food and Drug Administration (FDA) documentation.23,30
Johnson et al (2015) reported on the repeatability of invasive FFR.31 Data from 190 paired
assessments were analyzed (patients measured twice over 2 minutes). The test-retest coefficient
of variation of 2.5% (r2=98.2%) was reported using a “smart minimum” in the analyses (“the
lowest average of 5 consecutive cardiac cycles of sufficient quality within a run of 9 consecutive
quality beats”). Hulten and Di Carli (2015) noted that based on the Johnson results, an FFR of
0.8 would have a 95% confidence interval (CI) of 0.76 to 0.84.32 Gaur et al (2014) analyzed data
from 28 patients (58 vessels) with repeated FFR-CT and invasive FFR measurements.33 They
reported coefficients of variation of 3.4% (95% CI, 1.5% to 4.6%) for FFR-CT and 2.7% (95%
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 8 of 23
Selective Noninvasive Fractional Flow Reserve
CI, 1.8% to 3.3%) for invasive FFR. Although reproducibility was acceptable, whether test-retest
reliability over time might be similar is unclear.
The ability to obtain FFR-CT measurements is directly related to the quality of imaging data and
values are not calculated for small vessels (<1.8 mm). Nitrate administration is recommended
(generally standard practice unless contraindicated) for vasodilatation, and a lack of nitrates can
affect FFR-CT results. In addition, the FDA de novo summary lists factors that can adversely
impact FFR-CT results, including: imaging data quality, incorrect brachial pressure, myocardial
dysfunction and hypertrophy, and abnormal physiology (eg, congenital heart disease). Coronary
calcium might also impact measurements.34
Section Summary: Technical Performance
Reported results have indicated that the test-retest reliability is acceptable and other known
factors can impact variability of FFR-CT results.
Diagnostic Accuracy
Studies Included in FFR-CT Systematic Reviews: Per-Patient Diagnostic Accuracy
Twenty-six studies have contributed patient-level results to a 2015 meta-analysis that examined
5 non-FFR-CT imaging modalities (see Table 1).35 Five studies contributed results to 2 meta-
analyses, Wu et al (2016)36 and Danad et al (2017),37 evaluating the diagnostic accuracy of FFR-
CT using patients as the unit of analysis. Only the FDA-cleared HeartFlow software has been
evaluated prospectively across multiple sites. Two small retrospective studies have reported per-
patient performance characteristics for the prototype Siemens workstation-based software.38,39
The 3 HeartFlow FFRCT studies used successive software versions with reported improvement in
specificity (from 54% to 79%) between versions 1.2 and 1.4.24,27,40 The NXT Trial, the basis for
device clearance by FDA, was conducted at 11 sites in 8 countries (Canada, EU, Asia).27 Although
not examined in the 2 included meta-analyses, subgroup analyses suggested little variation in
results by sex and age.41 Effectively, the entirety of the data was obtained in patients of white or
Asian decent; almost all patients were appropriate for testing according to FDA clearance.
Danad et al
Danad et al (2017) included 23 studies published between January 2002 and February 2015
evaluating the diagnostic performance of CCTA, FFR-CT, SPECT, SECHO, MRI, or ICA compared
with an invasive FFR reference standard.37 The 3 included FFR-CT studies used the HeartFlow
software and had performed FFR in at least 75% of patients. A cutoff of 0.75 defined significant
stenosis in 8 (32%) studies and in the remainder 0.80 (the current standard used in all FFR-CT
studies). Per-patient and per-vessel meta-analyses were performed. Study quality was assessed
using QUADAS-242; no significant biases were identified in FFR-CT studies but a high risk of
biased patient selection was judged in 10 (43.4%) of other studies. HeartFlow funded publication
Open Access; 1 author was a consultant to, and another a cofounder of, HeartFlow.
On the patient level, MRI had the highest combined sensitivity (90%; 95% CI, 75% to 97%) and
specificity (94%; 95% CI, 79% to 99%) for invasive FFR, but were estimated from only 2 studies
(70 patients). FFR-CT had similar sensitivity (90%; 95% CI, 85% to 93%), but lower specificity
(71%; 95% CI, 65% to 75%), and accordingly a lower positive likelihood ratio (3.34; 95% CI,
1.78 to 6.25) than MRI (10.31; 95% CI, 3.14 to 33.9). The negative likelihood ratios were low
(lower is better) for both FFR-CT (0.16; 95% CI, 0.11 to 0.23) and MRI (0.12; 95% CI, 0.05 to
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 9 of 23
Selective Noninvasive Fractional Flow Reserve
0.30); however, the confidence interval is more narrow for FFR-CT due to larger sample for FFR-
CT. CCTA had a slightly higher negative likelihood ratio (0.22; 95% CI, 0.10 to 0.50). Results for
the per-vessel area under the summary receiver operating characteristic curve were similar
except for CCTA where per-patient results were considerably worse (eg, C statistic of 0.57 vs.
0.85). Reviewers noted heterogeneity in many estimates (eg, CCTA sensitivity, I2=80%). Finally,
pooled results for some imaging tests included few studies.
Wu et al
Wu et al (2016) identified 7 studies (833 patients, 1377 vessels) comparing FFR-CT with
invasively measured FFR from searches of PubMed, Cochrane, EMBASE, Medion, and meeting
abstracts through January 2016.36 Studies included patients with established or suspected SIHD.
In addition to the 3 FFR-CT studies pooled by Danad et al, 1 additional study using HeartFlow
technique (44 patients; 48 vessels) and 3 additional studies (180 patients; 279 vessels) using
Siemens cFFR software (not FDA approved or cleared) were identified. An invasive FFR cutoff of
0.80 was the reference standard in all studies. Per-patient results reported in 5 studies were
pooled and reported in Table 1. All studies were rated at low risk of bias and without applicability
concerns using the QUADAS-2 tool.42 Appropriate bivariate meta-analyses (accounting for
correlated sensitivity and specificity) were used.
As expected given study overlap, FFR-CT performance characteristics were similar to those
reported by Danad et al, but with a slightly higher specificity (see Table 1). The pooled per-vessel
C statistic was lower (0.86) than the per-patient result (0.90). No evidence of publication bias
was detected, but the number of studies was too small to adequately assess. Reviewers noted
that, in 2 studies, FFR-CT results were uninterpretable in 12.0%27 and 8.2%43 of participants.
Takx et al
Takx et al (2015) identified studies reporting on the ability of perfusion computed tomography
(CT), MRI, SECHO, PET, and SPECT to detect hemodynamically significant CAD as measured by
ICA with invasive FFR.35 Studies published through May 2014 were eligible for inclusion; PubMed,
EMBASE, and Web of Science were searched. QUADAS-2 was used to assess study quality42;
studies generally rated poorly on blinding of the index test result from the assessor and study
population selection. Reviewers designated the negative likelihood ratio as the diagnostic
characteristic of interest (ie, ability to exclude disease) noting that MPI (eg, MRI, SPECT, PET, or
CT) has been proposed to be a gatekeeper to ICA. No funding was obtained for the review and
the study was registered on PROSPERO44 (the 2 other meta-analyses were not).
The pooled negative likelihood ratios for MRI, PET, and perfusion CT were similar in magnitude
(0.12 to 0.14; see Table 1) although the confidence interval for PET was wide. Heterogeneity
among studies included in the pooled patient-level results was considered high for PET (I2=84%),
moderate for CT (I2=70%) and SPECT (I2=55%), and low for MRI (I2=0%) and SECHO (I2=0%).
Publication bias, when able to be assessed, was not suspected. With respect to ability to detect
hemodynamically significant ischemia, reviewers concluded that “MPI with MRI, CT, or PET has
the potential to serve as a gatekeeper for invasive assessment of hemodynamic significance by
ICA and FFR.” Studies of FFR-CT were not included in the analysis.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 10 of 23
Selective Noninvasive Fractional Flow Reserve
Table 1. Pooled Per-Patient Pooled Diagnostic Performance of Noninvasive Tests for Invasive
FFR
Sensitivity
Specificity
Test Studies N
(95% CI)
(95% CI)
C
LR+ (95% CI)
LR- (95% CI)
Danad et al (2017)37
MRI
2
70
90% (75 to 97)
94% (79 to 99)
0.94
10.3 (3.14 to 33.9)
0.12 (0.05 to 0.30)
FFR-CT
3
609
90% (85 to 93)
71% (65 to 75)
0.94
3.3 (1.78 to 6.25)
0.16 (0.11 to 0.23)
CCTA
4
694
90% (86 to 93)
39% (34 to 44)
0.57
1.5 (1.25 to 1.90)
0.22 (0.10 to 0.50)
SECHO
2
115
77% (61 to 88)
75% (63 to 85)
0.82
3.0 (1.94 to 4.65)
0.34 (0.17 to 0.66)
SPECT
3
110
70% (59 to 80)
78% (68 to 87)
0.79
3.4 (1.04 to 11.1)
0.40 (0.19 to 0.83)
ICA
2
954
69% (65 to 75)
67% (63 to 71)
0.75
2.5 (1.25 to 5.13)
0.46 (0.39 to 0.55)
Wu et al (2016)36
FFR-CT
5
833
89% (85 to 93)
76% (64 to 84)
0.90
3.7 (2.41 to 5.61)
0.14 (0.09 to 0.21)
Takx et al (2015)35
MRI
10
798
89% (86 to 92)
87% (83 to 90)
0.94
6.3 (4.88 to 8.12)
0.14 (0.10 to 0.18)
PCT
5
316
88% (82 to 92)
80% (73 to 86)
0.93
3.8 (1.94 to 7.40)
0.12 (0.04 to 0.33)
SECHO
4
177
69% (56 to 79)
84% (75 to 90)
0.83
3.7 (1.89 to 7.15)
0.42 (0.30 to 0.59)
SPECT
8
533
74% (67 to 79)
79% (74 to 83)
0.82
3.1 (2.09 to 4.70)
0.39 (0.27 to 0.55)
PET
2
224
84% (75 to 91)
87% (80 to 92)
0.93
6.5 (2.83 to 15.1)
0.14 (0.02 to 0.87)
CCTA: coronary computed tomography angiography; CI: confidence interval; FFR-CT: fractional flow reserve using coronary computed
tomography angiography; ICA: invasive coronary angiography; LR: likelihood ratio; MRI: magnetic resonance imaging; PCT: perfusion
computed tomography; PET: positron emission tomography; SECHO: stress echocardiography; SPECT: single-photon emission
computed tomography.
Section Summary: Diagnostic Accuracy
Three studies including 609 patients have evaluated diagnostic accuracy of the FDA-cleared
HeartFlow software. Software used in successive studies was also revised to improve
performance characteristics, particularly specificity. For example, using an earlier software
version, the DeFACTO Trial reported a specificity of 54%.45 Accordingly, pooled results from the
Danad systematic review must be interpreted carefully. In addition, there is some uncertainty in
the generalizability of results obtained in these studies conducted under likely controlled
conditions (eg, data from the NXT Trial27 forming the basis for FDA clearance).
Given the purpose to avoid ICA, the negative likelihood ratio, or how a negative result might
dissuade a clinician from proceeding to ICA, is of primary interest—ie, excluding a patient with
vessels having a high FFR from ICA. While confidence intervals are relatively wide and
overlapping, the negative likelihood ratio estimates of FFR-CT for excluding physiologically
significant coronary stenoses tended to be lower (ie, better) than CCTA alone, SECHO, SPECT,
and ICA. Only MRI yielded a similarly low or lower negative likelihood ratio than FFR-CT.
Clinical Utility
Indirect Evidence
Diagnostic performance can offer indirect evidence of clinical utility, assuming providers act
according to a test result. As previously noted, an effective gatekeeper strategy must be able to
decrease the probability of disease (rule out) sufficiently that a planned ICA would not be
performed. Ruling out disease is a function of the negative likelihood ratio that defines the
degree to which a negative test decreases the posttest odds (and probability) of disease. The
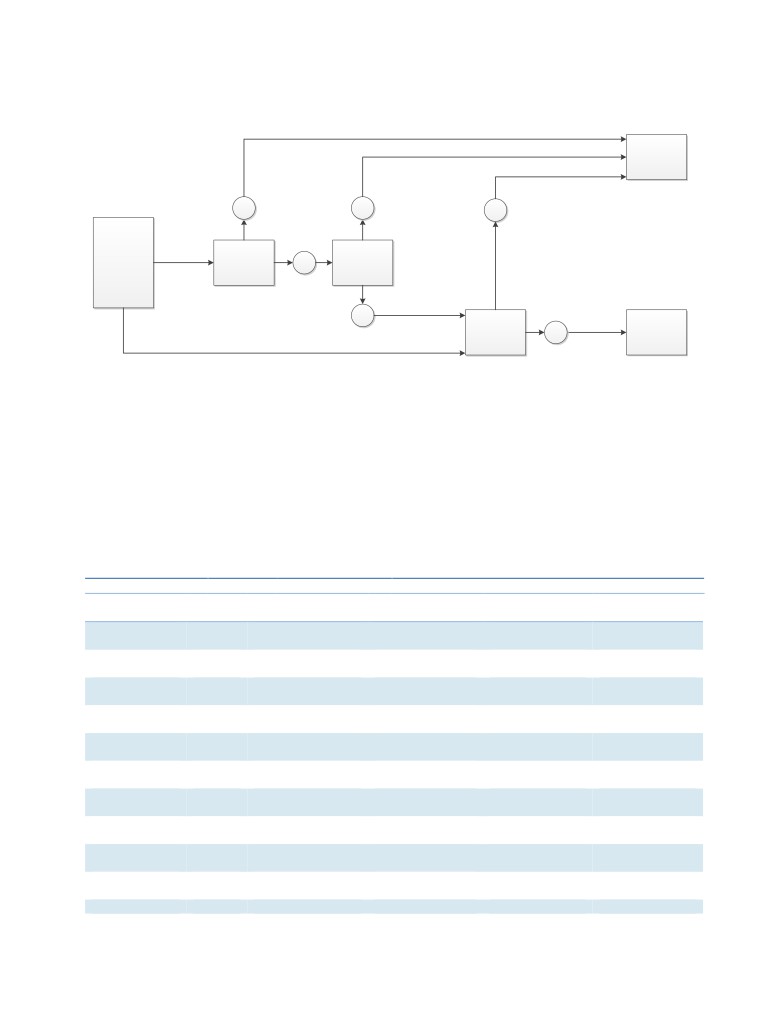
steps in the logic are illustrated in Figure 1.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information
Coronary Computed Tomography Angiography with
Page 11 of 23
Selective Noninvasive Fractional Flow Reserve
Figure 1. Pathway for Clinical Use of FFR-CT to Support Clinical Utility
Optimal Medical
Therapy
Low Negative
Likelihood Ratio
identifies additional
individuals with low
disease probability
-
-
who may avoid
-
Invasive Coronary
Stable Chest Pain
Angiography
with Intermediate
Risk of Coronary
Artery Disease Being
Coronary Computed
Considered for
Tomography
+
Add FFR-CT
Invasive Coronary
Angiography
Angiography
(CCTA)
(ie, Suspected Stable
Ischemic Heart
Disease)
+
Invasive Coronary
Obstructive
Angiography
Coronary Artery
(with invasive FFR if
+
Disease and
needed)
Revascularization
FFR-CT: fractional flow reserve using coronary computed tomography angiography.
Table 2 illustrates how a negative test would lower the probability of a hemodynamically
significant obstruction from pretest probabilities of 0.25, 0.50, or 0.75 for the various tests
examined in the meta-analyses. For example, according to the results of Danad et al, if the
pretest probability was 0.50, following a negative CCTA study the posttest probability would be
0.18 (95% CI, 0.09 to 0.33); and following a negative SECHO, 0.25 (95% CI, 0.15 to 0.40) or
SPECT, 0.29 (95% CI, 0.16 to 0.45). In contrast, beginning with a pretest probability of 0.50, a
negative FFR-CT would yield a posttest probability of 0.14 (95% CI, 0.10 to 0.19) (Danad et al)
and 0.12 (95% CI, 0.08 to 0.17) (Wu et al). Overall, the negative likelihood ratios and posttest
probability estimates for FFR-CT are slightly better than CCTA as well as SECHO and SPECT.
Table 2. Change in Disease Probability Following a Negative Test
Posttest Probability (95% CI) After Negative Test
Study
Modality
Negative LR
Pretest Probability
Pretest Probability
Pretest Probability
(95% CI)
0.25
0.50
0.75
Danad et al
(2016)
MRI
0.12 (0.05 to 0.30)
0.04 (0.02 to 0.09)
0.11 (0.05 to
0.26 (0.13 to
0.23)
0.47)
FFR-CT
0.16 (0.11 to 0.23)
0.05 (0.04 to 0.07)
0.14 (0.10 to
0.32 (0.25 to
0.19)
0.41)
CCTA
0.22 (0.10 to 0.50)
0.07 (0.03 to 0.14)
0.18 (0.09 to
0.40 (0.23 to
0.33)
0.60)
SECHO
0.34 (0.17 to 0.66)
0.10 (0.05 to 0.18)
0.25 (0.15 to
0.50 (0.34 to
0.40)
0.66)
SPECT
0.40 (0.19 to 0.83)
0.12 (0.06 to 0.22)
0.29 (0.16 to
0.55 (0.36 to
0.45)
0.71)
ICA
0.46 (0.39 to 0.55)
0.13 (0.12 to 0.15)
0.32 (0.28 to
0.58 (0.54 to
0.35)
0.62)
Wu et al
(2016)
FFR-CT
0.14 (0.09 to 0.21)
0.04 (0.03 to 0.07)
0.12 (0.08 to
0.30 (0.21 to
0.17)
0.39)
Takx et al
(2015)
MRI
0.14 (0.10 to 0.18)
0.04 (0.03 to 0.06)
0.12 (0.09 to
0.30 (0.23 to
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 12 of 23
Selective Noninvasive Fractional Flow Reserve
Posttest Probability (95% CI) After Negative Test
Study
Modality
Negative LR
Pretest Probability
Pretest Probability
Pretest Probability
(95% CI)
0.25
0.50
0.75
0.15)
0.35)
Perfusion
0.12 (0.04 to 0.33)
0.04 (0.01 to 0.10)
0.11 (0.04 to
0.26 (0.11 to
CT
0.25)
0.50)
SECHO
0.42 (0.30 to 0.59)
0.12 (0.09 to 0.16)
0.30 (0.23 to
0.56 (0.47 to
0.37)
0.64)
SPECT
0.39 (0.27 to 0.55)
0.12 (0.08 to 0.15)
0.28 (0.21 to
0.54 (0.45 to
0.35)
0.62)
PET
0.14 (0.02 to 0.87)
0.04 (0.01 to 0.22)
0.12 (0.02 to
0.30 (0.06 to
0.47)
0.72)
CCTA: coronary computed tomography angiography; CI: confidence interval; CT: computed tomography; FFR-CT: fractional flow
reserve using coronary computed tomography angiography; ICA: invasive coronary angiography; LR: likelihood ratio; MRI: magnetic
resonance imaging; PET: positron emission tomography; SECHO: stress echocardiography; SPECT: single-photon emission computed
tomography.
We identified 1 study (Curzen et al, 2016) that examined 200 consecutive individuals selected
from the NXT trial population “to reproduce the methodology of the invasive RIPCORD study”
with elective management of stable chest pain.46 All subjects received CCTA including FFR-CT “in
at least 1 vessel with diameter ≥ 2 mm and diameter stenosis ≥ 30%” as well as ICA within 60
days of CCTA. Three experienced interventional cardiologists reviewed the CCTA results (initially
without the FFR-CT results) and selected a management plan from the following 4 options: “1)
optimal medical therapy (OMT) alone; 2) PCI + OMT; 3) coronary artery bypass graft + OMT; or
4) more information about ischemia required - they committed to option 1 by consensus.”
Following the initial decision, results from the FFR-CT were shared with the same group of
interventional cardiologists who again made a decision by consensus based on the same 4
options. A cutoff of 0.80 or less was considered significant on FFR-CT. A stenosis was considered
significant on CCTA or ICA with 50% or more diameter narrowing. Change in management
between the first decision based on CCTA only and the second decision based on CCTA plus FFR-
CT was the primary end point of this study. Secondary end points included analysis of the vessels
considered to have significant stenosis based on CCTA alone versus CCTA plus FFR-CT as well as
vessels identified as targets for revascularization based on CCTA alone versus CCTA plus FFR-CT.
This study was conducted by investigators in the United Kingdom and Denmark. Funding was
provided by HeartFlow and multiple authors reported receiving fees, grants, and/or support from
HeartFlow.
Results for the primary end point (see Table 3) yielded a change in management category for 72
of 200 (36%) individuals. For the 87 individuals initially assigned to PCI based on CCTA alone,
the addition of the FFR-CT results shifted management for 26 of 87 (30%) to OMT (ie, no
ischemic lesion on FFR-CT) and an additional 16 (18%) individuals remained in the PCI category
but FFR-CT identified a different target vessel for PCI. These findings provide supportive
information that the improved diagnostic accuracy of FFR-CT in particular related to its better
negative likelihood ratio compared to CCTA alone would likely lead to changes in management
that would be expected to improve health outcomes.
Table 3. Summary of Overall Changes to Management in Patients Using CCTA vs CCTA + FFR-CT
Management Category Consensus
CCTA Alone,
CCTA + FFR-CT,
Strategy Changea
Decision
n (%)
n (%)
(95% CI)
More data required
38 (19.0%)
0
−
Optimal medical therapy
67 (33.5%)
113 (56.5%)
23% (18% to 29%)
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 13 of 23
Selective Noninvasive Fractional Flow Reserve
Management Category Consensus
CCTA Alone,
CCTA + FFR-CT,
Strategy Changea
Decision
n (%)
n (%)
(95% CI)
Percutaneous coronary intervention
87 (43.5%)
78 (39.0%)
-5% (-2% to -8%)
Coronary artery bypass graft surgery
8 (4.0%)
9 (4.5%)
0.5% (0.1% to 3%)
CCTA: coronary computed tomography angiography; CI: confidence interval; FFR-CT: fractional flow reserve using coronary computed
tomography angiography.
a p<0.001 for between-group change, CCTA alone vs CCTA + FFR-CT.
Direct Evidence
We identified 2 prospective comparative studies including 1 prospective nonrandomized study
that compared an FFR-CT strategy (CCTA with noninvasive FFR measurement when requested or
indicated) with ICA and 1 randomized controlled trial that examined CCTA as a gatekeeper to ICA
(see Tables 4 and 5). In addition, we identified 1 prospective cohort study and 2 retrospective
cohort studies of patients referred for CCTA, which included FFR-CT evaluation.
PLATFORM Study: The Prospective Longitudinal Trial of FFRCT: Outcome and Resource Impacts
(PLATFORM) study compared diagnostic strategies with or without FFR-CT in patients with
suspected stable angina but without known CAD.47,48 The study was conducted at 11 EU sites. All
testing was nonemergent. Patients were divided into 2 strata, according to whether the test
planned prior to study enrollment was: (1) noninvasive or (2) ICA (the patient population of
interest in this evidence review). Patients were enrolled in consecutive cohorts, with the first
cohort undergoing a usual care strategy followed by a second cohort provided CCTA with FFR-CT
performed when requested (recommended if stenoses ≥30% were identified). Follow-up was
scheduled at 90 days and 6 and 12 months after entry (99.5% of patients had 1-year follow-up
data). Funding was provided by HeartFlow and multiple authors reported receiving fees, grants,
and/or support from HeartFlow. Data analyses were performed by the Duke Clinical Research
Institute.
ICA without obstructive disease at 90 days was the primary end point in patients with planned
invasive testing—“no stenosis ≥ 50% by core laboratory quantitative analysis or invasive FFR <
0.80.” Secondary end points included ICA without obstructive disease following planned
noninvasive testing, and (1) MACE at 1 year defined as a composite of all-cause mortality,
myocardial infarction (MI), and urgent revascularization and (2) MACE and vascular events within
14 days. Quality of life (QOL) was evaluated using the Seattle Angina Questionnaire, and EQ-5D
(5-item and 100-point visual analog scale). CCTA studies were interpreted by site investigators;
quantitative coronary angiography measurements were performed at a central laboratory, as was
FFR-CT. Cumulative radiation was also assessed. A sample size of 380 patients in the invasive
strata yielded a 90% power to detect a 50% decrease in the primary end point given a 30%
event rate (ICA without obstructive disease) with a usual care strategy and a dropout rate up to
10%.
ICA was planned in 380 participants, of whom 193 (50.8%) had undergone prior noninvasive
testing. The mean pretest probability in the planned ICA strata was approximately 50% (51.7%
and 49.4% in the 2 groups). FFR-CT was requested in 134 patients and successfully obtained in
117 of 134 (87.3%) in the FFR-CT group. At 90 days, 73.3% of those in the usual care group had
no obstructive findings on ICA compared with 12.4% in the FFR-CT group based on core
laboratory readings (56.7% and 9.3% based on site readings). The difference was similar in a
propensity-matched analysis of a subset of participants (n=148 from each group or 78% of the
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 14 of 23
Selective Noninvasive Fractional Flow Reserve
entire sample). Prior noninvasive testing did not appear associated with nonobstructive findings.
MACE rates were low and did not differ between strategies. Mean level of radiation exposure
though 1 year was also similar in the usual care group (10.4 mSv) and the planned ICA group
(10.7 mSv). No differences in QOL were found between groups.49
Results of the PLATFORM study support the notion that, in patients with planned ICA, FFR-CT
can decrease the rate of ICAs and unnecessary procedures (finding no significant obstructive
disease) and that FFR-CT may provide clinically useful information to physicians and patients.
Study limitations include a nonrandomized design; high rate of no obstructive disease with a
usual care strategy (73.3%), which was higher than the 30% rate assumed in the sample size
estimates; and a sample size that was small with respect to evaluating adverse cardiac events.
Although finding a large effect in patients with planned invasive testing, the nonrandomized
design limits causal inferences and certainty that the magnitude of effect. The propensity-
matched analysis (in a matched subset) offers some reassurance, but the sample size was likely
too small to provide robust results.
CAD-Man Trial: Dewey et al (2016) conducted the Coronary Artery Disease Management (CAD-
Man) trial, a single-center, parallel-group assignment trial examining CCTA as a gatekeeper to
ICA in patients with atypical angina or chest pain and suspected CAD who were indicated for
ICA.50 Patients were randomized to direct ICA or to ICA only if a prior CCTA was positive (a
stenosis ≥70% stenosis in any vessel or ≥50% in the left main coronary artery). The trialists
reported that when obstructive disease was suspect following CCTA, late enhancement MRI was
performed to evaluate the extent of viable myocardium (completed in 17 patients) to guide
revascularization; however, the study protocol clarified that MRI was not used for decisions to
proceed to ICA. A major procedural complication (death, stroke, MI, or event requiring >24-hour
hospitalization) within 24 hours was the primary outcome; secondary outcomes included ICA with
obstructive CAD (diagnostic yield), revascularizations, and MACE during long-term follow-up. The
trial was performed in Germany. Patients were excluded if they had evidence of ischemia or signs
of MI and just over half (56.5%) were inpatients at the time of enrollment. Obstructive disease
was defined as “at least one 50% diameter stenosis in the left main coronary artery or at least
one 70% diameter stenosis in other coronary arteries.” Allocation concealment appeared
adequate, but the trial was unblinded owing to the nature of the intervention. In addition, the
mean pretest probability of CAD at baseline was higher in the ICA-only arm (37.3% vs. 31.3%;
see Table 4). The research was supported by public funding.
ICAs were reduced by 85.6% in the CCTA arm and by 80.9% for ICA with no obstructive disease.
A major procedural complication (the primary outcome) occurred in a single patient undergoing
CCTA. PCIs were less frequent when CCTA was performed—9.6% versus 14.2% (p<0.001). Over
a median follow-up of 3.3 years, MACE rates were similar in the trial arms (4.2% in the CCTA
group vs 3.7% with ICA; adjusted hazard ratio [HR], 0.90; 95% CI, 0.30 to 2.69). In the CCTA
arm, there was 1 death, 2 patients with unstable angina, and 6 revascularizations; in the ICA arm
there was 1 MI, 1 stroke, and 5 revascularizations.
The trial demonstrated that CCTA as a gatekeeper to planned ICA can avoid a large number of
procedures, a corresponding increase in the diagnostic yield, and fewer revascularizations. Of
note, the prevalence of obstructive CAD found on ICA in this study population was 13% (43/334
eligible for primary outcome analysis), which is lower than the prevalence of obstructive CAD in
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 15 of 23
Selective Noninvasive Fractional Flow Reserve
the PLATFORM population (26.7%). Thus, the subset of individuals who went onto ICA following
CCTA findings of obstructive CAD was 20 (12%) of 167 eligible for primary outcome analysis and
only 3 (1.7%) were found to have no obstructive CAD on ICA. MACE rates did not differ between
arms. The trial was powered neither to detect a difference nor to assess noninferiority—
implications of the absence of a difference are limited. Finally, although the patient population
included those scheduled for elective ICA, it was heterogeneous, including those with recent
onset and longer standing chest pain. The single-center nature of the trial is an additional
limitation; a subsequent multicenter trial (DISCHARGE) is ongoing.
Table 4. Characteristics of Comparative Studies
Characteristics
Nonrandomized
Randomized
PLATFORM
CAD-Man
ICA
FFR-CT
ICA
CCTA
(n=187)
(n=193)
(n=162)
(n=167)
Age (SD), y
63.4
(10.9)
60.7
(10.2)
60.4
(11.4)
60.4
(11.3)
Female, n (%)
79 (42.2%)
74 (38.3%)
88 (52.7%)
78 (48.1%)
Race/ethnic minority, n (%)
2 (1.1%)
1 (0.5%)
Pretest probability obstructive CAD, %
51.7%
49.4%
(17.2%)
37.3%
31.3%
Angina (%)
Typical
52 (27.8%)
45 (23.3%)
Atypical
122 (65.2%)
142 (73.6%)
79 (48.8%)
65 (38.9%)
Noncardiac
12 (7.0%)
5 (2.6%)
80 (49.4%)
97 (58.1%)
Other chest discomfort
3 (1.8%)
5 (3.0%)
Prior noninvasive testing, n (%)
92 (49.2%)
101 (52.3%)
84 (50.3%)
92 (56.8%)
Diabetes, n (%)
36 (19.3%)
30 (15.5%)
30 (18.5%)
15 (9.0%)
Current smoker
34 (21.0%)
41 (24.5%)
Current or past smoker
103 (55.1%)
101 (52.3%)
85 (52.4%)
88 (52.6%)
CAD: coronary artery disease; CCTA: coronary computed tomography angiography; FFR-CT: fractional flow reserve using coronary
computed tomography angiography; ICA: invasive coronary angiography.
Table 5. Results of Comparative Studies
Outcomes
Nonrandomized
Randomized
PLATFORM
CAD-Man
ICA
FFR-CT
ICA
CCTA
(n=187)
(n=193)
(n=162)
(n=167)
Noninvasive FFR-CT
Requested, n (%)
134 (69.4%)
Successfully performed, n (%)
117 (60.1%)
ICA no obstructive disease, n (%)
137 (73.3%)
24 (12.4%)
137 (84.5%)
6 (3.6%)
Absolute difference (95% CI), %
60.8% (53.0% to 68.7%)
80.9% (74.6% to 87.2%)
ICA, n (%)
187 (100%)
76 (39.4%)
162 (100%)
24 (14.4%)
Absolute difference (95% CI), %
60.6% (53.7% to 67.5%)
85.6% (80.3% to 90.9%)
Revascularization, n (%)
PCI
49 (26.2%)
55 (28.5%)
CABG
18 (9.6%)
10 (5.2%)
Any
67 (35.8%)
65 (33.7%)
23 (14.2%)
16 (9.6%)
1-year outcomes, n (%)
MACEa
2 (1.1%)
2 (1.0%)
MACEb
6 (3.7%)
7 (4.2%)
CABG: coronary artery bypass grafting; CI: confidence interval; FFR-CT: fractional flow reserve using coronary computed tomography
angiography; ICA: invasive coronary angiography; MACE: major adverse cardiovascular events; PCI: percutaneous coronary
intervention.
a Death, myocardial infarction, unplanned urgent revascularization
b Cardiac death, myocardial infarction, stroke, unstable angina, any revascularization.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 16 of 23
Selective Noninvasive Fractional Flow Reserve
Møller Jensen et al Prospective Cohort: Møller Jensen et al (2017) reported on a single-institution
study of 774 consecutive individuals with suspicion of CAD referred for nonemergent ICA or
CCTA.51 Subjects were analyzed in 2 groups: a low-intermediate-risk group accounting for 76%
of patients with mean pretest probability of CAD 31% and a high-risk group accounting for 24%
of patients with mean pretest probability of CAD 67%. Among the 745 who received CCTA, FFR-
CT was selectively ordered in 28% of patients overall (23% in the low-intermediate-risk group,
41% in the high-risk group). CCTA was considered inconclusive in 3% of subjects and among
those with conclusive CCTA, FFR-CT yielded few inconclusive results, with less than 3% of cases.
During a minimum 90-day follow-up, the combined testing strategy of selective FFR-CT following
CCTA resulted in avoiding ICA in 91% of low-intermediate-risk and 75% of high-risk individuals.
None of the patients who avoided ICA based on CCTA with selective FFR-CT were associated with
serious clinical adverse events over an average of 157 days of follow-up.
Nørgaard et al Retrospective Cohort: Nørgaard et al (2017) reported on results from symptomatic
patients referred for CCTA at a single center in Denmark from May 2014 to April 2015.52 All data
were obtained from medical records and registries; the study was described as a “review” of
diagnostic evaluations and apparently retrospectively conducted. Follow-up through 6 to 18
months was ascertained. From 1248 referred patients, 1173 underwent CCTA; 858 received
medical therapy, 82 underwent ICA, 44 MPI, and 189 FFR-CT (185 [98%] obtained successfully).
Of the 185 individuals who successfully obtained FFR-CT, FFR-CT demonstrated values of 0.80 or
less in 1 or more vessels in 57 (31%) patients and 49 (86%) went on to ICA; whereas of the 128
with higher FFR-CT values, only 5 (4%) went on to ICA. Assuming ICA was planned for all
patients undergoing FFR-CT, these results are consistent with FFR-CT being able to decrease the
rate of ICA. However, implications are limited by the retrospective design, performance at a
single center, and lack of a comparator arm including one for CCTA alone.
Lu et al Retrospective Cohort: Lu et al (2017) retrospectively examined a subgroup referred to
ICA53 from the completed PROspective Multicenter Imaging Study for Evaluation of Chest Pain
(PROMISE) trial. PROMISE was a pragmatic trial comparing CCTA with functional testing for the
initial evaluation of patients with suspected SIHD.54 Of 550 participants referred to ICA within 90
days, 279 were not considered for the analyses due to CCTA performed without nitroglycerin
(n=139), CCTA not meeting slice thickness guidelines (n=90), or nondiagnostic studies (n=50).
Of the remaining 271 patients, 90 scans were inadequate to obtain FFR-CT, leaving 181 (33%) of
those referred to ICA for analysis. Compared with those excluded, patients in the analytic sample
were less often obese, hypertensive, diabetic, minority, or reported a CAD equivalent symptom.
The 2 groups had similar pretest probabilities of disease, revascularization rates, and MACE, but
the distribution of stenoses in the analytic sample tended to be milder (p=0.06). FFR-CT studies
were performed in a blinded manner and not available during the conduct of PROMISE for
decision making.
Severe stenoses (≥70%) or left main disease (≥50%) were present in 110 (66%) patients by
CCTA result and in 54% by ICA. Over a 29-month median follow-up, MACE (death, nonfatal MI,
hospitalization for unstable angina) or revascularization occurred in 51% of patients (9% MACE,
49% revascularization). A majority (72%) of the sample had at least 1 vessel with an FFR-CT
≤0.80, which was also associated with a higher risk of revascularization but with a wide
confidence interval (HR = 5.1; 95% CI, 2.6 to 11.5). If reserved for patients with an FFR-CT of
0.80 or less, ICAs might have been avoided in 50 patients (ie, reduced by 28%) and the rate of
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 17 of 23
Selective Noninvasive Fractional Flow Reserve
ICA without 50% or more stenosis from 27% (calculated 95% CI, 21% to 34%) to 15%
(calculated 95% CI, 10% to 23%). If the 90 patients whose images sent for FFR-CT but were
unsatisfactory proceeded to ICA—as would have occurred in practice—the rate of ICA might have
decreased by 18% and ICA without significant stenosis from 31% to 25%.
The authors suggested that when CCTA is used as the initial evaluation for patients with
suspected SIHD, adding FFR-CT could have decreased the referral rate to ICA in PROMISE from
12.2% to 9.5%, or close to the 8.1% rate observed in the PROMISE functional testing arm. They
also noted similarity of their findings to PLATFORM and concluded, “In this hypothesis-generating
study of patients with stable chest pain referred to ICA after [C]CTA, we found that adding
FFRCT may improve the efficiency of referral to ICA, addressing a major concern of an anatomic
[C]CTA strategy. FFRCT has incremental value over anatomic [C]CTA in predicting
revascularization or major adverse cardiovascular events.”
This retrospective observational subgroup analysis from PROMISE suggests that when CCTA is
the initial noninvasive test for the evaluation of suspected SIHD, FFR-CT prior to ICA has the
potential to reduce unnecessary ICAs and increase the diagnostic yield. However, study
limitations and potential generalizability are important to consider. First, analyses included only a
third of CCTA patients referred to ICA and the some characteristics of the excluded group
differed from the analytic sample. Second, conclusions assume that an FFR-CT greater than 0.80
will always dissuade a physician from recommending ICA and even in the presence of severe
stenosis (eg, ≥70% in any vessel or ≥50% in the left main)—or almost half (46%) of patients
with an FFR-CT greater than 0.80. Finally, estimates including patients with either nondiagnostic
CCTA studies (n=50) or studies inadequate for calculating FFR-CT (n=90) are more appropriate
because most likely those patients would proceed in practice to ICA. Accordingly, the estimates
are appropriately considered upper bounds for what might be seen in practice. It is also
important to note that in strata of the PLATFORM trial enrolling patients for initial noninvasive
testing (not planned ICA), ICA was more common following CCTA and contingent FFR-CT than
following usual care (18.3% vs. 12.0%) and ICA, with no obstructive disease more frequent in
the FFR-CT arm (12.5% vs. 6.0%).
Section Summary: Clinical Utility
The evidence on the diagnostic performance characteristics, particularly showing higher
specificity of FFR-CT and better negative likelihood ratio as compared to CCTA alone, may be
combined with indirect evidence that CCTA with a selective FFR-CT strategy would likely lead to
changes in management that would be expected to improve health outcomes, particularly by
limiting unnecessary invasive coronary angiography testing. Moreover, there is direct evidence,
provided by 2 prospective and 2 retrospective studies, that compares health outcomes observed
during 90-day to 1-year follow-up for strategies using CCTA particularly in combination with
selective FFR-CT with strategies using ICA or other noninvasive imaging tests. The available
evidence provides support that use of CCTA with selective FFR-CT is likely to reduce the use of
ICA in individuals with stable chest pain who are unlikely to benefit from revascularization by
demonstrating the absence of functionally significant obstructive CAD. In addition, the benefits
are likely to outweigh potential harms given that rates of revascularization for functionally
significant obstructive CAD appear to be similar and cardiac-related adverse events do not appear
to be increased following a CCTA with selective FFR-CT strategy. While individual studies are
noted to have specific methodologic limitations and some variation is noted in the magnitude of
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 18 of 23
Selective Noninvasive Fractional Flow Reserve
benefit across studies, in aggregate the evidence provides reasonable support that the selective
addition of FFR-CT following CCTA results in a meaningful improvement in the net health
outcome.
SUMMARY OF EVIDENCE
For individuals with stable chest pain at intermediate risk of coronary artery disease (CAD; ie,
suspected or presumed stable ischemic heart disease) being considered for invasive coronary
angiography (ICA) who receive noninvasive fractional flow reserve (FFR) measurement following
positive coronary computed tomography angiography (CCTA), the evidence includes both direct
and indirect evidence: 2 meta-analyses on diagnostic performance; 1 prospective, multicenter
nonrandomized comparative study; 1 prospective cohort; 2 retrospective cohort studies; and a
study reporting changes in management associated with CCTA-based strategies with selective
addition of fractional flow reserve using coronary computed tomography angiography (FFR-CT)
and a randomized controlled trial (RCT) of CCTA alone compared with ICA. Relevant outcomes
are test accuracy and validity, morbid events, quality of life, resource utilization, and treatment-
related morbidity. The meta-analyses indicated that CCTA has high sensitivity but moderately low
specificity for hemodynamically significant obstructive disease. Given the available evidence that
CCTA alone has been used to select patients to avoid ICA, the studies showing higher specificity
of FFR-CT and lower negative likelihood ratio of FFR-CT compared with CCTA alone, may be used
to build a chain of evidence that CCTA with a selective FFR-CT strategy would likely lead to
changes in management that would be expected to improve health outcomes by further limiting
unnecessary ICA testing. Moreover, there is direct evidence, provided by 2 prospective and 2
retrospective studies, that compares health outcomes observed during 90-day to 1-year follow-up
for strategies using CCTA particularly in combination with selective FFR-CT with strategies using
ICA or other noninvasive imaging tests. The available evidence provides support that use of CCTA
with selective FFR-CT is likely to reduce the use of ICA in individuals with stable chest pain who
are unlikely to benefit from revascularization by demonstrating the absence of functionally
significant obstructive CAD. In addition, the benefits are likely to outweigh potential harms
because rates of revascularization for functionally significant obstructive CAD appear to be similar
and treatment-related adverse events do not appear to increase following CCTA with a selective
FFR-CT strategy. While individual studies are noted to have specific methodologic limitations and
some variation has been noted in the magnitude of benefit across studies, in aggregate the
evidence provides reasonable support that the selective addition of FFR-CT following CCTA
results in a meaningful improvement in the net health outcome. The evidence is sufficient to
determine that the technology results in meaningful improvements in the net health outcome.
PRACTICE GUIDELINES AND POSITION STATEMENTS
National Institute for Health and Care Excellence
In 2017, the National Institute for Health and Care Excellence endorsed fractional flow reserve
using coronary computed tomography angiography (FFR-CT), with the following conclusions:
“The committee concluded that the evidence suggests that HeartFlow FFRCT is safe, has high
diagnostic accuracy, and that its use may avoid the need for invasive investigations.”55
Recommendations included:
•
“The case for adopting HeartFlow FFR-CT for estimating fractional flow reserve from
coronary CT angiography (CCTA) is supported by the evidence. The technology is non-
invasive and safe, and has a high level of diagnostic accuracy.”
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 19 of 23
Selective Noninvasive Fractional Flow Reserve
•
“HeartFlow FFR-CT should be considered as an option for patients with stable, recent
onset chest pain who are offered CCTA as part of the NICE pathway on chest pain. Using
HeartFlow FFR-CT may avoid the need for invasive coronary angiography and
revascularization. For correct use, HeartFlow FFR-CT requires access to 64-slice (or
above) CCTA facilities.”
U.S. PREVENTIVE SERVICES TASK FORCE RECOMMENDATIONS
Not applicable.
ONGOING AND UNPUBLISHED CLINICAL TRIALS
Some currently unpublished trials that might influence this review are listed in Table 6.
Table 6. Summary of Key Trials
NCT No.
Trial Name
Planned
Completion
Enrollment
Date
Ongoing
NCT02173275
Computed TomogRaphic Evaluation of Atherosclerotic DEtermiNants
618
Jul 2017
of Myocardial IsChEmia
NCT02400229
Diagnostic Imaging Strategies for Patients With Stable Chest Pain
3546
Sept 2019
and Intermediate Risk of Coronary Artery Disease: Comparative
Effectiveness Research of Existing Technologies) - A Pragmatic
Randomised Controlled Trial of CT Versus ICA
NCT02973126
Assessment of Fractional Flow reservE Computed Tomography
270
Oct 2020
Versus Single Photon Emission Computed Tomography in the
Diagnosis of Hemodynamically Significant Coronary Artery Disease.
(AFFECTS)
NCT02499679a Assessing Diagnostic Value of Non-invasive FFRCT in Coronary Care
5000
Feb 2021
(ADVANCE)
NCT02208388
Prospective Evaluation of MyocaRdial PerFUSion ComputEd
1000
Apr 2024
Tomography Trial
Unpublished
NCT01810198a Coronary Computed Tomographic Angiography for Selective Cardiac
1631
Mar 2016
Catheterization (CONSERVE)
(completed)
NCT02805621
Machine leArning Based CT angiograpHy derIved FFR: a Multi-
352
Jan 2017
ceNtEr, Registry
(completed)
NCT: national clinical trial.
a Denotes industry-sponsored or cosponsored trial.
CODING
The following codes for treatment and procedures applicable to this policy are included below
for informational purposes. Inclusion or exclusion of a procedure, diagnosis or device code(s)
does not constitute or imply member coverage or provider reimbursement. Please refer to the
member's contract benefits in effect at the time of service to determine coverage or non-
coverage of these services as it applies to an individual member.
CPT/HCPCS
75574
Computed tomographic angiography, heart, coronary arteries and bypass grafts
(when present), with contrast material, including 3D image postprocessing
(including evaluation of cardiac structure and morphology, assessment of cardiac
function, and evaluation of venous structures, if performed)
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information
Coronary Computed Tomography Angiography with
Page 20 of 23
Selective Noninvasive Fractional Flow Reserve
• As of publication of this policy, there is no specific CPT code for fractional flow reserve
using coronary computed tomographic angiography.
ICD-10 Diagnoses
I20.9
Angina pectoris, unspecified
I25.118
Atherosclerotic heart disease of native coronary artery with other forms of angina
pectoris
I25.119
Atherosclerotic heart disease of native coronary artery with unspecified angina
pectoris
REVISIONS
10-15-2017
Policy added to the bcbsks.com web site on 09-15-2017 with an effective date of
10-15-2017.
REFERENCES
1.
Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J
Med. Mar 11 2010;362(10):886-895. PMID 20220183
2.
Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable
coronary disease. N Engl J Med. Apr 12 2007;356(15):1503-1516. PMID 17387127
3.
Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the
diagnosis and management of patients with stable ischemic heart disease: a report of the American
College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and
the American College of Physicians, American Association for Thoracic Surgery, Preventive
Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and
Society of Thoracic Surgeons. J Am Coll Cardiol. Dec 18 2012;60(24):e44-e164. PMID 23182125
4.
Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery
disease. N Engl J Med. Jun 14 1979;300(24):1350-1358. PMID 440357
5.
Genders TS, Steyerberg EW, Alkadhi H, et al. A clinical prediction rule for the diagnosis of coronary
artery disease: validation, updating, and extension. Eur Heart J. Jun 2011;32(11):1316-1330. PMID
21367834
6.
Wasfy MM, Brady TJ, Abbara S, et al. Comparison of the Diamond-Forrester method and Duke
Clinical Score to predict obstructive coronary artery disease by computed tomographic angiography.
Am J Cardiol. Apr 01 2012;109(7):998-1004. PMID 22236462
7.
Versteylen MO, Joosen IA, Shaw LJ, et al. Comparison of Framingham, PROCAM, SCORE, and
Diamond Forrester to predict coronary atherosclerosis and cardiovascular events. J Nucl Cardiol. Oct
2011;18(5):904-911. PMID 21769703
8.
Min JK, Dunning A, Gransar H, et al. Medical history for prognostic risk assessment and diagnosis of
stable patients with suspected coronary artery disease. Am J Med. Aug 2015;128(8):871-878. PMID
25865923
9.
Genders TS, Steyerberg EW, Hunink MG, et al. Prediction model to estimate presence of coronary
artery disease: retrospective pooled analysis of existing cohorts. BMJ. Jun 12 2012;344:e3485. PMID
22692650
10.
CAD Consortium. Pre-test probability of CAD. 2016;
Accessed June 1, 2017.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 21 of 23
Selective Noninvasive Fractional Flow Reserve
11.
De Bruyne B, Fearon WF, Pijls NH, et al. Fractional flow reserve-guided PCI for stable coronary artery
disease. N Engl J Med. Sep 25 2014;371(13):1208-1217. PMID 25176289
12.
De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in
stable coronary disease. N Engl J Med. Sep 13 2012;367(11):991-1001. PMID 22924638
13.
Tonino PA, De Bruyne B, Pijls NH, et al. Fractional flow reserve versus angiography for guiding
percutaneous coronary intervention. N Engl J Med. Jan 15 2009;360(3):213-224. PMID 19144937
14.
Pothineni NV, Shah NS, Rochlani Y, et al. U.S. trends in inpatient utilization of fractional flow reserve
and percutaneous coronary intervention. J Am Coll Cardiol. Feb 16 2016;67(6):732-733. PMID
26868697
15.
Blue Cross Blue Shield Association Technology Evaluation Center (TEC). Fractional Flow Reserve and
Coronary Artery Revascularization. TEC Assessment. June 2011;26:Tab 2.
16.
Fearon WF, Shilane D, Pijls NH, et al. Cost-effectiveness of percutaneous coronary intervention in
patients with stable coronary artery disease and abnormal fractional flow reserve. Circulation. Sep 17
2013;128(12):1335-1340. PMID 23946263
17.
van Nunen LX, Zimmermann FM, Tonino PA, et al. Fractional flow reserve versus angiography for
guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a
randomised controlled trial. Lancet. Nov 7 2015;386(10006):1853-1860. PMID 26333474
18.
Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC guidelines on the management of stable
coronary artery disease: the Task Force on the management of stable coronary artery disease of the
European Society of Cardiology. Eur Heart J. Oct 2013;34(38):2949-3003. PMID 23996286
19.
Windecker S, Kolh P, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization:
The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the
European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of
the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. Oct 1
2014;35(37):2541-2619. PMID 25173339
20.
Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017
Appropriate Use Criteria for coronary revascularization in patients with stable ischemic heart disease:
a report of the American College of Cardiology Appropriate Use Criteria Task Force, American
Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography,
American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions,
Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll
Cardiol. May 02 2017;69(17):2212-2241. PMID 28291663
21.
Pijls NH, Van Gelder B, Van der Voort P, et al. Fractional flow reserve. A useful index to evaluate the
influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. Dec 1
1995;92(11):3183-3193. PMID 7586302
22.
de Bruyne B, Bartunek J, Sys SU, et al. Simultaneous coronary pressure and flow velocity
measurements in humans. Feasibility, reproducibility, and hemodynamic dependence of coronary
flow velocity reserve, hyperemic flow versus pressure slope index, and fractional flow reserve.
Circulation. Oct 15 1996;94(8):1842-1849. PMID 8873658
23.
HeartFlow. DEN130045, FFRct V. 1.4. 2013;
http://www.accessdata.fda.gov/cdrh_docs/reviews/DEN130045.pdf. Accessed September 11, 2016.
24.
Koo BK, Erglis A, Doh JH, et al. Diagnosis of ischemia-causing coronary stenoses by noninvasive
fractional flow reserve computed from coronary computed tomographic angiograms. Results from the
prospective multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via
Noninvasive Fractional Flow Reserve) study. J Am Coll Cardiol. Nov 1 2011;58(19):1989-1997. PMID
22032711
25.
Min JK, Koo BK, Erglis A, et al. Effect of image quality on diagnostic accuracy of noninvasive
fractional flow reserve: results from the prospective multicenter international DISCOVER-FLOW
study. J Cardiovasc Comput Tomogr. May-Jun 2012;6(3):191-199. PMID 22682261
26.
Nakazato R, Park HB, Berman DS, et al. Noninvasive fractional flow reserve derived from computed
tomography angiography for coronary lesions of intermediate stenosis severity: results from the
DeFACTO study. Circ Cardiovasc Imaging. Nov 2013;6(6):881-889. PMID 24081777
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 22 of 23
Selective Noninvasive Fractional Flow Reserve
27.
Nørgaard BL, Leipsic J, Gaur S, et al. Diagnostic performance of noninvasive fractional flow reserve
derived from coronary computed tomography angiography in suspected coronary artery disease: the
NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. Apr
1 2014;63(12):1145-1155. PMID 24486266
28.
Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography
for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. Jun 4
2013;61(22):2233-2241. PMID 23562923
29.
Kim KH, Doh JH, Koo BK, et al. A novel noninvasive technology for treatment planning using virtual
coronary stenting and computed tomography-derived computed fractional flow reserve. JACC
Cardiovasc Interv. Jan 2014;7(1):72-78. PMID 24332418
30.
HeartFlow. K152733, FFRct v2.0. 2016;
https://www.accessdata.fda.gov/cdrh_docs/pdf15/K152733.pdf. Accessed September 11, 2016.
31.
Johnson NP, Johnson DT, Kirkeeide RL, et al. Repeatability of fractional flow reserve despite
variations in systemic and coronary hemodynamics. JACC Cardiovasc Interv. Jul 2015;8(8):1018-
1027. PMID 26205441
32.
Hulten E, Di Carli MF. FFRCT: Solid PLATFORM or thin ice? J Am Coll Cardiol. Dec 1
2015;66(21):2324-2328. PMID 26475206
33.
Gaur S, Bezerra HG, Lassen JF, et al. Fractional flow reserve derived from coronary CT angiography:
variation of repeated analyses. J Cardiovasc Comput Tomogr. Jul-Aug 2014;8(4):307-314. PMID
25151923
34.
Nørgaard BL, Gaur S, Leipsic J, et al. Influence of coronary calcification on the diagnostic
performance of CT angiography derived FFR in coronary artery disease: a substudy of the NXT Trial.
JACC Cardiovasc Imaging. Sep 2015;8(9):1045-1055. PMID 26298072
35.
Takx RA, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging
compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ
Cardiovasc Imaging. Jan 2015;8(1). PMID 25596143
36.
Wu W, Pan DR, Foin N, et al. Noninvasive fractional flow reserve derived from coronary computed
tomography angiography for identification of ischemic lesions: a systematic review and meta-
analysis. Sci Rep. 2016;6:29409. PMID 27377422
37.
Danad I, Szymonifka J, Twisk JWR, et al. Diagnostic performance of cardiac imaging methods to
diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow
reserve as a reference standard: a meta-analysis. Eur Heart J. Apr 01 2017;38(13):991-998. PMID
27141095
38.
Renker M, Schoepf UJ, Wang R, et al. Comparison of diagnostic value of a novel noninvasive
coronary computed tomography angiography method versus standard coronary angiography for
assessing fractional flow reserve. Am J Cardiol. Nov 01 2014;114(9):1303-1308. PMID 25205628
39.
De Geer J, Sandstedt M, Bjorkholm A, et al. Software-based on-site estimation of fractional flow
reserve using standard coronary CT angiography data. Acta Radiol. Oct 2016;57(10):1186-1192.
PMID 26691914
40.
Min JK, Leipsic J, Pencina MJ, et al. Diagnostic accuracy of fractional flow reserve from anatomic CT
angiography. JAMA. Sep 26 2012;308(12):1237-1245. PMID 22922562
41.
Thompson AG, Raju R, Blanke P, et al. Diagnostic accuracy and discrimination of ischemia by
fractional flow reserve CT using a clinical use rule: results from the Determination of Fractional Flow
Reserve by Anatomic Computed Tomographic Angiography study. J Cardiovasc Comput Tomogr.
Mar-Apr 2015;9(2):120-128. PMID 25819194
42.
Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of
diagnostic accuracy studies. Ann Intern Med. Oct 18 2011;155(8):529-536. PMID 22007046
43.
Coenen A, Lubbers MM, Kurata A, et al. Fractional flow reserve computed from noninvasive CT
angiography data: diagnostic performance of an on-site clinician-operated computational fluid
dynamics algorithm. Radiology. Mar 2015;274(3):674-683. PMID 25322342
44.
PROSPERO. International prospective register of systematic reviews. n.d.;
https://www.crd.york.ac.uk/PROSPERO/. Accessed April 28, 2017.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information

Coronary Computed Tomography Angiography with
Page 23 of 23
Selective Noninvasive Fractional Flow Reserve
45.
Min JK, Berman DS, Budoff MJ, et al. Rationale and design of the DeFACTO (Determination of
Fractional Flow Reserve by Anatomic Computed Tomographic AngiOgraphy) study. J Cardiovasc
Comput Tomogr. Sep-Oct 2011;5(5):301-309. PMID 21930103
46.
Curzen NP, Nolan J, Zaman AG, et al. Does the routine availability of CT-derived FFR Influence
management of patients with stable chest pain compared to CT angiography alone?: The FFRCT
RIPCORD Study. JACC Cardiovasc Imaging. Oct 2016;9(10):1188-1194. PMID 27568119
47.
Douglas PS, De Bruyne B, Pontone G, et al. 1-year outcomes of FFRCT-guided care in patients with
suspected coronary disease: the PLATFORM Study. J Am Coll Cardiol. Aug 2 2016;68(5):435-445.
PMID 27470449
48.
Douglas PS, Pontone G, Hlatky MA, et al. Clinical outcomes of fractional flow reserve by computed
tomographic angiography-guided diagnostic strategies vs. usual care in patients with suspected
coronary artery disease: the prospective longitudinal trial of FFR(CT): outcome and resource impacts
study. Eur Heart J. Dec 14 2015;36(47):3359-3367. PMID 26330417
49.
Hlatky MA, De Bruyne B, Pontone G, et al. Quality-of-life and economic outcomes of assessing
fractional flow reserve with computed tomography angiography: PLATFORM. J Am Coll Cardiol. Dec 1
2015;66(21):2315-2323. PMID 26475205
50.
Dewey M, Rief M, Martus P, et al. Evaluation of computed tomography in patients with atypical
angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial.
Bmj. Oct 24 2016;355:i5441. PMID 27777234
51.
Moller Jensen J, Erik Botker H, Norling Mathiassen O, et al. Computed tomography derived fractional
flow reserve testing in stable patients with typical angina pectoris: influence on downstream rate of
invasive coronary angiography. Eur Heart J Cardiovasc Imaging. Apr 20 2017. PMID 28444153
52.
Nørgaard BL, Hjort J, Gaur S, et al. Clinical use of coronary CTA-derived FFR for decision-making in
stable CAD. JACC Cardiovasc Imaging. May 2017;10(5):541-550. PMID 27085447
53.
Lu MT, Ferencik M, Roberts RS, et al. Noninvasive FFR Derived From Coronary CT Angiography:
Management and Outcomes in the PROMISE Trial. JACC Cardiovasc Imaging. Apr 07 2017. PMID
28412436
54.
Douglas PS, Hoffmann U, Lee KL, et al. PROspective Multicenter Imaging Study for Evaluation of
chest pain: rationale and design of the PROMISE trial. Am Heart J. Jun 2014;167(6):796-803 e791.
PMID 24890527
55.
National Institute for Health and Care Excellence. HeartFlow FFRCT for estimating fractional flow
reserve from coronary CT angiography [MTG32]. 2017; https://www.nice.org.uk/guidance/mtg32.
Accessed April 28, 2017.
Current Procedural Terminology © American Medical Association. All Rights Reserved.
Contains Public Information